Selected Lectures in Organic and Bioorganic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Degradation of Cyclohexane and Cyclohexanone by Bacillus Lentus Strain LP32
Vol. 12(47), pp. 6632-6635, 20 November, 2013 DOI: 10.5897/AJB2013.13137 ISSN 1684-5315 ©2013 Academic Journals African Journal of Biotechnology http://www.academicjournals.org/AJB Full Length Research Paper Degradation of cyclohexane and cyclohexanone by Bacillus lentus strain LP32 Bolanle O. Opere, Oluwafemi S. Obayori* and Adebanji A. Raji Department of Microbiology, Lagos State University, Ojo, Lagos, Nigeria. Accepted 10 October, 2013 A Gram-positive bacterium, Bacillus lentus LP32, originally isolated on the basis of its ability to utilise pyrene as sole source of carbon was found to be able to grow luxuriantly on alicyclic compounds as sole substrates. It showed poor growth on anthracene, naphthalene, 1-naphthol and phenanthrene. Growth rate on cyclohexane was 1.32 d-1, while doubling time was 0.76 d. The corresponding values for growth on cyclohexanone were 0.77 d-1 and 1.29 d, respectively. Within 10 days, the amount of cyclohexane in culture reduced from 317.62 to 102.55 mgl-1, then to 23.04 mgl-1 on day 18. On cyclohexanone, substrate concentration decreased from 287.56 mgl-1 to 101.66 mgl-1 in 10 days before declining to 24.21 mgl-1 on day 18. The rate of degradation when growing on cyclohexane was 23.50 mgl-1d-1 in the first 10 days and 9.93 mgl-1d-1 between day 10 and day 18, with 67.71% degradation in 10 days and overall percentage degradation of 92.43%. On cyclohexanone, the corresponding values were 18.59 and 9.68 mg l-1d-1 as well as 64.65 and 91.58%, respectively. -
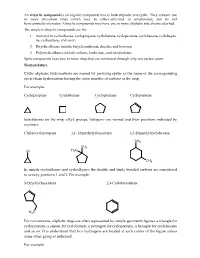
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

An Analysis of Electrophilic Aromatic Substitution: a “Complex Approach” PCCP
Volume 23 Number 9 7 March 2021 Pages 5033–5682 PCCP Physical Chemistry Chemical Physics rsc.li/pccp ISSN 1463-9076 PERSPECTIVE Janez Cerkovnik et al . An analysis of electrophilic aromatic substitution: a “complex approach” PCCP View Article Online PERSPECTIVE View Journal | View Issue An analysis of electrophilic aromatic substitution: a ‘‘complex approach’’† Cite this: Phys. Chem. Chem. Phys., a a b 2021, 23, 5051 Nikola Stamenkovic´, Natasˇa Poklar Ulrih and Janez Cerkovnik * Electrophilic aromatic substitution (EAS) is one of the most widely researched transforms in synthetic organic chemistry. Numerous studies have been carried out to provide an understanding of the nature of its reactivity pattern. There is now a need for a concise and general, but detailed and up-to-date, overview. The basic principles behind EAS are essential to our understanding of what the mechanisms underlying EAS are. To date, textbook overviews of EAS have provided little information about the Received 5th October 2020, mechanistic pathways and chemical species involved. In this review, the aim is to gather and present the Accepted 21st December 2020 up-to-date information relating to reactivity in EAS, with the implication that some of the key concepts DOI: 10.1039/d0cp05245k will be discussed in a scientifically concise manner. In addition, the information presented herein suggests certain new possibilities to advance EAS theory, with particular emphasis on the role of modern Creative Commons Attribution-NonCommercial 3.0 Unported Licence. rsc.li/pccp -

Chemistry Courses 2005-2006
Chemistry Courses 2005-2006 Autumn 2005 Chem 11101 General Chemistry I, Variant A Lee Chem 11102 General Chemistry I, Variant B Norris Chem 12200 Honors General Chemistry I Levy Chem 22000 Organic Chemistry I Yu Chem 22300 Intermediate Organic Chemistry Mrksich Chem 26100 Quantum Mechanics Mazziotti Chem 30100 Advanced Inorganic Chemistry Hopkins Chem 30900 Bioinorganic Chemistry He Chem 32100 Physical Organic Chemistry I Ismagilov Chem 32200 Organic Synthesis and Structure Rawal Chem 32600 Protein Fundamentals Piccirilli Chem 36100 Wave Mechanics & Spectroscopy Butler Chem 36400 Chemical Thermodynamics Dinner Winter 2006 Chem 11201 General Chemistry II, Variant A Scherer Chem 11202 General Chemistry II, Variant B Butler Chem 12300 Honors General Chemistry II Dinner Chem 20100 Inorganic Chemistry I Hillhouse Chem 22100 Organic Chemistry II Rawal Chem 23100 Honors Organic Chemistry II Kozmin Chem 26200 Thermodynamics Norris Chem 26700 Experimental Physical Chemistry Levy Chem 30200 Synthesis & Physical Methods in Inorganic Chemistry Jordan Chem 30400 Organometallic Chemistry Bosnich Chem 32300 Tactics of Organic Synthesis Yamamoto Chem 32400 Physical Organic Chemistry II Mrksich Chem 36200 Quantum Mechanics Freed Chem 36300 Statistical Mechanics Mazziotti Chem 38700 Biophysical Chemistry Lee Spring 2006 Chem 11301 General Chemistry III, Variant A Kozmin Chem 11302 General Chemistry III, Variant B Guyot-Sionnest Chem 20200 Inorganic Chemistry II Jordan Chem 22200 Organic Chemistry III Kent Chem 23200 Honors Organic Chemistry III Yamamoto Chem 22700 Advanced Organic / Inorganic Laboratory (8 students) He Chem 26300 Chemical Kinetics and Dynamics Sibner Chem 26800 Computational Chemistry & Biology Freed Chem 30600 Chemistry of the Elements Hillhouse Chem 31100 Supramolecular Chemistry Bosnich Chem 32500 Bioorganic Chemistry Piccirilli Chem 32900 Polymer Chemistry Yu Chem 33000 Complex Systems Ismagilov Chem 36500 Chemical Dynamics Scherer Chem 36800 Advanced Computational Chemistry & Biology Freed . -

Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
CH. 13 Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution Electrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. Recall: R3 R4 E Y E Y C C + E Y R1 C C R4 R1 C C R4 − R2 R1 δ+ δ R2 R3 R2 R3 In aromatic rings, however, we see substitution of one of the benzene ring hydrogens for an electrophile. H E + E Y + Y H δ+ δ− The mechanism is the same regardless of the electrophile. It involves two steps: (1) Addition of the electrophile to form a high-energy carbocation. (2) Elimination of the proton to restore the aromatic ring system. H H H H slow E Y + Y step 1 + E δ+ δ− high energy arenium ion H H step 2 fast E E + Y + Y H The first step is the slow step since the aromaticity of the benzene ring system is destroyed on formation of the arenium ion intermediate. This is a high energy species but it is stabilized by resonance with the remaining two double bonds. The second step is very fast since it restores the aromatic stabilization. 1 CH. 13 H H H H H H E E E There are five electrophilic aromatic substitution reactions that we will study. (1) Nitration H NO2 H2SO4 + HNO3 (2) Sulfonation H SO3H + H2SO4 (3) Halogenation with bromine or chlorine H X FeX3 X = Br, Cl + X2 (4) Friedel-Crafts Alkylation H R AlX + RX 3 (5) Friedel-Crafts Acylation O H O C AlX3 R + Cl C R 2 CH. -

Curriculum of Department of Pharmacy (6-Year Program)
Curriculum of Department of Pharmacy (6-year program) 1st year 2nd year 3rd year 4th year 5th year 6th year Basic Subjects for Pharmacy Student; Lectures and Practices of Basic Specialized Lectures and Practices Drug Therapy and its Related Practical Training and Beginning of Research for Graduation and SGL and more Sciences Research for Graduation Advanced Lectures Fundamental Education Professional Education Ⅰ Professional Education Ⅱ ■Humanism ■Humanism ■Preparatory Pharmacy Education ■Professional Pharmacy Education ■Practical Training ■Advanced Education Introduction to Humanism I Introduction to Humanism Ⅱ English for Pharmacy Oriental Medicine Preparation for Practical Training Research for Graduation Pharmacotherapeutics Practical Training in Hospital Advanced Clinical Training ■Preparatory Pharmacy Education ■Professional Pharmacy Education Drug Information Science Practical Training in Community Phrarmacy General Pharmacy Practice II ■Introduction of Pharmacy Basic English and English for Pharmacy Clinical Chemistry Drug Development and Production Japanese Traditional Medicine Invitation to Pharmacy Basic Statistics Synthesis of Target Molecules Pharmacy and Society ■Advanced Education Clinical Pharmacokinetics Early Exposure to Pharmacy Practical Trainings for Pharmacy Bioorganic Chemistry General Pharmacy Practice Research for Graduation Clinical Drug Evaluation Students Pharmaceutical Health Care Training Medical Economy Natural Products Chemistry ■Practical Training Education Drug Safty Evaluation and Pharmacoepidemiology -

Recreating Ancient Metabolic Pathways Before Enzymes Kamila B
Recreating ancient metabolic pathways before enzymes Kamila B. Muchowska, Elodie Chevallot-Beroux, Joseph Moran To cite this version: Kamila B. Muchowska, Elodie Chevallot-Beroux, Joseph Moran. Recreating ancient metabolic path- ways before enzymes. Bioorganic and Medicinal Chemistry, Elsevier, 2019, 27 (12), pp.2292-2297. 10.1016/j.bmc.2019.03.012. hal-02516541 HAL Id: hal-02516541 https://hal.archives-ouvertes.fr/hal-02516541 Submitted on 23 Mar 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Graphical Abstract To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered. Recreating ancient metabolic pathways Leave this area blank for abstract info. before enzymes Kamila B. Muchowskaa* , Elodie Chevallot-Berouxa, and Joseph Morana* University of Strasbourg, CNRS, ISIS UMR 7006, 67000 Strasbourg, France Bioorganic & Medicinal Chemistry journal homepage: www.elsevier.com Recreating ancient metabolic pathways before enzymes Kamila B. Muchowska,a* Elodie Chevallot-Beroux,a and Joseph Morana* a University of Strasbourg, CNRS, ISIS UMR 7006, 67000 Strasbourg, France. ARTICLE INFO ABSTRACT Article history: The biochemistry of all living organisms uses complex, enzyme-catalyzed metabolic reaction Received networks. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing -

Enantiomers & Diastereomers
Chapter 5 Stereochemistry Chiral Molecules Ch. 5 - 1 1. Chirality & Stereochemistry An object is achiral (not chiral) if the object and its mirror image are identical Ch. 5 - 2 A chiral object is one that cannot be superposed on its mirror image Ch. 5 - 3 1A. The Biological Significance of Chirality Chiral molecules are molecules that cannot be superimposable with their mirror images O O ● One enantiomer NH causes birth defects, N O the other cures morning sickness O Thalidomide Ch. 5 - 4 HO NH HO OMe Tretoquinol OMe OMe ● One enantiomer is a bronchodilator, the other inhibits platelet aggregation Ch. 5 - 5 66% of all drugs in development are chiral, 51% are being studied as a single enantiomer Of the $475 billion in world-wide sales of formulated pharmaceutical products in 2008, $205 billion was attributable to single enantiomer drugs Ch. 5 - 6 2. Isomerisom: Constitutional Isomers & Stereoisomers 2A. Constitutional Isomers Isomers: different compounds that have the same molecular formula ● Constitutional isomers: isomers that have the same molecular formula but different connectivity – their atoms are connected in a different order Ch. 5 - 7 Examples Molecular Constitutional Formula Isomers C4H10 and Butane 2-Methylpropane Cl Cl C3H7Cl and 1-Chloropropane 2-Chloropropane Ch. 5 - 8 Examples Molecular Constitutional Formula Isomers CH O CH C H O OH and 3 3 2 6 Ethanol Methoxymethane O OCH and 3 C H O OH 4 8 2 O Butanoic acid Methyl propanoate Ch. 5 - 9 2B. Stereoisomers Stereoisomers are NOT constitutional isomers Stereoisomers have their atoms connected in the same sequence but they differ in the arrangement of their atoms in space. -

Unit 3 – Stereochemistry
Stereochemistry Stereochemistry refers to the 3-dimensional properties and reactions of molecules. It has its own language and terms that need to be learned in order to fully communicate and understand the concepts. New vocabulary and concepts Handedness Chirality Fischer Projections Depicting Asymmetric Carbons (R) and (S) Nomenclature Enantiomers Diastereomers Optical Activity Stereochemistry Isomers: Different compounds that have the same molecular formula (composition) but different connectivity. Two classes: - Structural (constitutional) isomers: same molecular formula but different bonding sequence - Stereoisomers: same molecular formula, same bonding sequence, but different arrangement in space. Handedness…..Chirality Handedness” right glove doesn’t fit the left hand. Superimposable: A term that describes the ability to precisely overlap one object over another. Only identical objects are superposable, everything else is non-superposable superimposable nonsuperimposable Chiral molecules & Chirality Center Chemical substances can be handed, and they are called chiral. Chiral Molecules: are molecules that are nonsuperimposable on their mirror image. A carbon atom that is bonded to four chiral carbon atom different groups is called chairal carbon atom or stereocenter (asymmetric carbon atom). It is sp3 carbon and labeled with a strict. Achiral: A molecule is achiral if it is superimposable on its mirror image H H Cl Cl Practices on Asymmetric Carbons Example: Identify all asymmetric carbons present in the following compounds. Br Br H OH H H CH2CH3 H C *C C C H Br CH3 * H H H H H H H H H3C Br CH3 * * OH * CH CHCOOH* 3 * * * Fischer Projections: ➢ It is a two-dimensional representation of a three-dimensional organic molecule by projection. -

Chapter 4: Stereochemistry Introduction to Stereochemistry
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane Me Me Me C Me H Bu Bu Me Me 2-methylhexane H H mirror Me rotate Bu Me H 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane H C Et Et Me Pr Pr 3-methylhexane Me Me H H mirror Et rotate H Me Pr 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane .Compounds that are not superimposable with their mirror image are called chiral (in Greek, chiral means "handed") 3-methylhexane is a chiral molecule. .Compounds that are superimposable with their mirror image are called achiral. 2-methylhexane is an achiral molecule. .An atom (usually carbon) with 4 different substituents is called a stereogenic center or stereocenter. Enantiomers Et Et Pr Pr Me CH3 Me H H 3-methylhexane mirror enantiomers Et Et Pr Pr Me Me Me H H Me H H Two compounds that are non-superimposable mirror images (the two "hands") are called enantiomers. Introduction To Stereochemistry Structural (constitutional) Isomers - Compounds of the same molecular formula with different connectivity (structure, constitution) 2-methylpentane 3-methylpentane Conformational Isomers - Compounds of the same structure that differ in rotation around one or more single bonds Me Me H H H Me H H H H Me H Configurational Isomers or Stereoisomers - Compounds of the same structure that differ in one or more aspects of stereochemistry (how groups are oriented in space - enantiomers or diastereomers) We need a a way to describe the stereochemistry! Me H H Me 3-methylhexane 3-methylhexane The CIP System Revisited 1. -

Nucleophilic Aromatic Substitution on Aryl-Amido Ligands Promoted by Oxidizing Osmium(IV) Centers
Inorg. Chem. 2004, 43, 5804−5815 Nucleophilic Aromatic Substitution on Aryl-Amido Ligands Promoted by Oxidizing Osmium(IV) Centers Jake D. Soper, Erik Saganic, David Weinberg, David A. Hrovat, Jason B. Benedict,† Werner Kaminsky,† and James M. Mayer* Department of Chemistry, Campus Box 351700, UniVersity of Washington, Seattle, Washington 98195-1700 Received January 30, 2004 IV Addition of amine nucleophiles to acetonitrile solutions of the Os anilido complex TpOs(NHPh)Cl2 (1) [Tp ) hydrotris(1-pyrazolyl)borate] gives products with derivatized anilido ligands, i.e., TpOs[NH-p-C6H4(N(CH2)5)]Cl2 (2) from piperidine and TpOs[NH-p-C6H4N(CH2)4]Cl2 (3) from pyrrolidine. These materials are formed in ∼30% yield III under anaerobic conditions, together with ∼60% yields of the Os aniline complex TpOs(NH2Ph)Cl2 (5). Formation of the para-substituted materials 2 or 3 from 1 involves oxidative removal of two hydrogen atoms (two H+ and two - e ). The oxidation can be accomplished by 1, forming 5,orbyO2. Related reactions have been observed with other amines and with the 2-naphthylamido derivative, which gives an ortho-substituted product. Kinetic studies indicate an addition−elimination mechanism involving initial attack of the amine nucleophile on the anilido ligand. These are unusual examples of nucleophilic aromatic substitution of hydrogen. Ab initio calculations on 1 show that the LUMO has significant density at the ortho and para positions of the anilido ligand, resembling the LUMO of nitrobenzene. By analogy with nucleophilic aromatic substitution, 2 is quantitatively formed from piperidine and IV the p-chloroanilide TpOs(NH-p-C6H4Cl)Cl2 (7).