Sodium Aluminium Silicate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suppression Mechanisms of Alkali Metal Compounds
SUPPRESSION MECHANISMS OF ALKALI METAL COMPOUNDS Bradley A. Williams and James W. Fleming Chemistry Division, Code 61x5 US Naval Research Lnhoratory Washington, DC 20375-5342, USA INTRODUCTION Alkali metal compounds, particularly those of sodium and potassium, are widely used as fire suppressants. Of particular note is that small NuHCOi particles have been found to be 2-4 times more effective by mass than Halon 1301 in extinguishing both eountertlow flames [ I] and cup- burner flames [?]. Furthermore, studies in our laboratory have found that potassium bicarbonate is some 2.5 times more efficient by weight at suppression than sodium bicarhonatc. The primary limitation associated with the use of alkali metal compounds is dispersal. since all known compounds have very low volatility and must he delivered to the fire either as powders or in (usually aqueous) solution. Although powders based on alkali metals have been used for many years, their mode of effective- ness has not generally been agreed upon. Thermal effects [3],namely, the vaporization of the particles as well as radiative energy transfer out of the flame. and both homogeneous (gas phase) and heterogeneous (surface) chemistry have been postulated as mechanisms by which alkali metals suppress fires [4]. Complicating these issues is the fact that for powders, particle size and morphology have been found to affect the suppression properties significantly [I]. In addition to sodium and potassium, other alkali metals have been studied, albeit to a consider- ably lesser extent. The general finding is that the suppression effectiveness increases with atomic weight: potassium is more effective than sodium, which is in turn more effective than lithium [4]. -

Reac on of Oxides with Acids and Bases
Reacon of oxides with acids and bases Reacon of oxides with acids and bases Lesson plan (Polish) Lesson plan (English) Reacon of oxides with acids and bases Source: domena publiczna. Link to the lesson Before you start you should know that salts are a group of chemical compounds of ionic structure, composed of cations + of metals (or a cation of ammonium with the following formula NH4 ) and anions of the acid radical; that the names of salts are made up of two parts: acid radical name and metal name; that some metal oxides, such as sodium oxide or calcium oxide, react with water and produce hydroxides as a result of this reaction; that some oxides of non‐metals react with water to form acids, e.g. sulfur dioxide; that the red cabbage stock turns red in solutions of acids, violet in neutral solutions, and green in base solutions; that the methyl orange is coloured red in acids. You will learn to describe, by means of equations, the obtainment of salts in reactions of certain metal oxides with acids as well as some non‐metal oxides with bases. Nagranie dostępne na portalu epodreczniki.pl Source: GroMar Sp. z o.o.. Nagranie dźwiękowe abstraktu Reacons of metal oxides with acids Task 1 Before conducng experiment, formulate research queson and hypothesis. Write down observaon and conclusions. Analysis of the experiment: Does calcium oxide react with hydrochloric acid? Research queson Hypothesis Experiment 1 Research problem Does calcium oxide react with hydrochloric acid? Hypothesis Select one of the presented hypotheses and then verify it. The calcium oxide reacts with the hydrochloric acid. -

United States Patent Office Patented Jan
3,071,480 United States Patent Office Patented Jan. 1, 1963 2 actually can be modified as to proportion within the range 3,071,480 of approximately 60% by weight (62.1 mol percent) to GLASS COMPOSTEON FOR BEADS Charles E. Searigiat and Ezra M. Alexander, Jackson, 68% by weight (69.9 mol percent) and to this we add Miss, assignors to Cataphote Corporation, Toledo, basically three ingredients; these three ingredients being Ohio, a corporation of Ohio sodium oxide in a preferred quantity of 14% by weight No Drawing. Filed Dec. 5, 1960, Ser. No. 73,552 (14 mol percent) but which may range in quantity from 4. Claims. (C. 106-52) approximately 12.7% by weight (12.7 mol percent) to 16% by weight (16 mol percent), calcium oxide in a pre This invention relates to an improved glass composition ferred quantity of 14% by weight (15.5 mol percent), and, more particularly, to a glass composition suitable O but which may range in quantity from 12.7% by weight for the manufacture of solid spherical glass beads. (14.0 mol percent) to 16% by weight (17.7 mol percent), In the manufacture of glass beads for use in reflective and barium oxide in a preferred quantity of 6.3% by road signs, reflective road and curve markings, and the weight (2.5 mol percent), but which may range in quan like, it is of paramount importance that the glass com tity from 5.9% by weight (2.3 mol percent) to 7.3% by position meet certain rigid requirements, both as to 5 weight (3.0 mol percent). -

Sodium Aluminium Silicate (Tentative)
SODIUM ALUMINIUM SILICATE (TENTATIVE) th Prepared at the 80 JECFA and published in FAO JECFA Monographs 17 (2015), superseding tentative specifications prepared at the 77th JECFA (2013) and published in FAO JECFA Monographs 14 (2013). An ADI 'not specified' for silicon dioxide and certain silicates was established at the 29th JECFA (1985). A PTWI of 2 mg/kg bw for total aluminium was established at the 74th JECFA (2011). The PTWI applies to all aluminium compounds in food, including food additives. Information required: Functional uses other than anticaking agent, if any, and information on the types of products in which it is used and the use levels in these products Data on solubility using the procedure documented in the “Compendium of Food Additives Specifications, Vol. 4, Analytical methods” Data on the impurities soluble in 0.5 M hydrochloric acid, from a minimum of five batches. If a different extraction and determination method is used, provide data along with details of method and QC data. Suitability of the analytical method for the determination of aluminium, silicon and sodium using the proposed “Method of assay” along with data, from a minimum of five batches, using the proposed method. If a different method is used, provide data along with details of the method and QC data. SYNONYMS Sodium silicoaluminate; sodium aluminosilicate; aluminium sodium silicate; silicic acid, aluminium sodium salt; INS No. 554 DEFINITION Sodium aluminium silicate is a series of amorphous hydrated sodium aluminium silicates with varying proportions of Na2O, Al2O3 and SiO2. It is manufactured by, precipitation process, reacting aluminium sulphate and sodium silicate. -

What Is the Value of Water Contact Angle on Silicon?
materials Article What Is the Value of Water Contact Angle on Silicon? Paweł Bryk 1, Emil Korczeniewski 2, Grzegorz S. Szyma ´nski 2, Piotr Kowalczyk 3, Konrad Terpiłowski 4 and Artur P. Terzyk 2,* 1 Department of Chemistry, Chair of Theoretical Chemistry, Maria Curie-Skłodowska University, 20-031 Lublin, Poland; [email protected] 2 Faculty of Chemistry, Physicochemistry of Carbon Materials Research Group, Nicolaus Copernicus University in Toru´n,Gagarin Street 7, 87-100 Toru´n,Poland; [email protected] (E.K.); [email protected] (G.S.S.) 3 College of Science, Health, Engineering and Education, Murdoch University, Murdoch WA 6150, Australia; [email protected] 4 Department of Chemistry, Chair of Physical Chemistry of Interfacial Phenomena, Maria Curie-Skłodowska University, 20-031 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-61-14-371 Received: 4 March 2020; Accepted: 26 March 2020; Published: 27 March 2020 Abstract: Silicon is a widely applied material and the wetting of silicon surface is an important phenomenon. However, contradictions in the literature appear considering the value of the water contact angle (WCA). The purpose of this study is to present a holistic experimental and theoretical approach to the WCA determination. To do this, we checked the chemical composition of the silicon (1,0,0) surface by using the X-ray photoelectron spectroscopy (XPS) method, and next this surface was purified using different cleaning methods. As it was proved that airborne hydrocarbons change a solid wetting properties the WCA values were measured in hydrocarbons atmosphere. Next, molecular dynamics (MD) simulations were performed to determine the mechanism of wetting in this atmosphere and to propose the force field parameters for silica wetting simulation. -

MAGNESIUM SILICATE, Synthetic
MAGNESIUM SILICATE, synthetic Prepared at the 74th JECFA (2011) and published in FAO Monographs 11 (2011) superseding specifications prepared at the 61st JECFA (2003), published in the Combined Compendium of Food Additive Specifications, FAO JECFA Monographs 1 (2005). An ADI ‘not specified’ was established at the 25th JECFA (1981). SYNONYMS INS No. 553(i) DEFINITION Magnesium silicate (synthetic) is manufactured by the precipitation reaction between sodium silicate and a soluble magnesium salt. The aqueous suspension of the precipitate is filtered and the collected solid washed, dried, classified for particle size and packaged. The finest material is intended for use as an anticaking agent and the coarser particles are for use as a filtering aid. The moisture content of the material meant for use as an anticaking agent is kept to less than 15%. Although magnesium silicate is of variable composition, the molar ratio of MgO to SiO2 is approximately 2:5. Chemical name Magnesium silicate C.A.S. number 1343-88-0 Assay Not less than 15% of MgO and not less than 67% of SiO2, calculated on the ignited basis. DESCRIPTION Very fine, white, odourless powder, free from grittiness FUNCTIONAL USES Anticaking agent, filtering aid CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Insoluble in water pH (Vol. 4) 7.0-11.0 (1 in 10 slurry) Magnesium (Vol. 4) Mix about 0.5 g of the sample with 10 ml of dilute hydrochloric acid TS, filter, and neutralize the filtrate to litmus paper with ammonia TS. The neutralized filtrate gives a positive test for magnesium. Silicate Prepare a bead by fusing a few crystals of sodium ammonium phosphate on a platinum loop in the flame of a Bunsen burner. -

Periodic Activity of Metals Periodic Trends and the Properties of the Elements SCIENTIFIC
Periodic Activity of Metals Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Elements are classified based on similarities, differences, and trends in their properties, including their chemical reactions. The reactions of alkali and alkaline earth metals with water are pretty spectacular chemical reactions. Mixtures bubble and boil, fizz and hiss, and may even smoke and burn. Introduce the study of the periodic table and periodic trends with this exciting demonstration of the activity of metals. Concepts • Alkali and alkaline earth metals • Periodic table and trends • Physical and chemical properties • Metal activity Materials Calcium turnings, Ca, 0.3 g Beaker, Berzelius (tall-form), Pyrex®, 500-mL, 4 Lithium metal, Li, precut piece Forceps or tongs Magnesium ribbon, Mg, 3-cm Knife (optional) Sodium metal, Na, precut piece Petri dishes, disposable, 4 Phenolphthalein, 1% solution, 2 mL Scissors Water, distilled or deionized, 600 mL Safety Precautions Lithium and sodium are flammable, water-reactive, corrosive solids; dangerous when exposed to heat or flame. They react violently with water to produce flammable hydrogen gas and solutions of corrosive metal hydroxides. Hydrogen gas may be released in sufficient quantities to cause ignition. Do NOT “scale up” this demonstration using larger pieces of sodium or lithium! These metals are shipped in dry mineral oil. Store them in mineral oil until immediately before use. Do not allow these metals to stand exposed to air from one class period to another or for extended periods of time. Purchasing small, pre-cut pieces of lithium and sodium greatly reduces their potential hazard. Calcium metal is flammable in finely divided form and reacts upon contact with water to give flammable hydrogen gas and corrosive calcium hydroxide. -

Mercury and Mercury Compounds
United States Office of Air Quality EPA-454/R-97-012 Environmental Protection Planning And Standards Agency Research Triangle Park, NC 27711 December 1997 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF MERCURY AND MERCURY COMPOUNDS L & E EPA-454/R-97-012 Locating And Estimating Air Emissions From Sources of Mercury and Mercury Compounds Office of Air Quality Planning and Standards Office of Air and Radiation U.S. Environmental Protection Agency Research Triangle Park, NC 27711 December 1997 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation for use. EPA-454/R-97-012 TABLE OF CONTENTS Section Page EXECUTIVE SUMMARY ................................................ xi 1.0 PURPOSE OF DOCUMENT .............................................. 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS ................................. 2-1 3.0 BACKGROUND ........................................................ 3-1 3.1 NATURE OF THE POLLUTANT ..................................... 3-1 3.2 OVERVIEW OF PRODUCTION, USE, AND EMISSIONS ................. 3-1 3.2.1 Production .................................................. 3-1 3.2.2 End-Use .................................................... 3-3 3.2.3 Emissions ................................................... 3-6 4.0 EMISSIONS FROM MERCURY PRODUCTION ............................. 4-1 4.1 PRIMARY MERCURY -
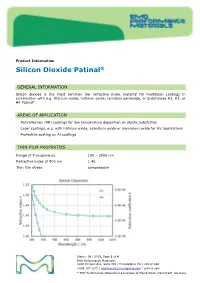
Sio2,Silicon Dioxide Patinal®Us
Product Information Silicon Dioxide Patinal ® GENERAL INFORMATION Silicon dioxide is the most common low refractive index material for multilayer coatings in combination with e.g. titanium oxide, hafnium oxide, tantalum pentoxide, or Substances H1, H2, or H4 Patinal . AREAS OF APPLICATION • Antireflection (AR) coatings for low temperature deposition on plastic substrates • Laser coatings, e.g. with hafnium oxide, scandium oxide or aluminium oxide for UV applications • Protective coating on Al-coatings THIN FILM PROPERTIES Range of Transparency 190 – 2000 nm Refractive index at 500 nm 1.46 Thin film stress compressive Status: 08 / 2019, Page 1 of 4 EMD Performance Materials* 1200 Intrepid Ave, Suite 300 / Philadelphia, PA / 19112 USA +888 367 3275 / [email protected] / patinal.com * EMD Performance Materials is a business of Merck KGaA, Darmstadt, Germany wavl / nm 200 250 350 500 650 800 1000 1250 n 1.551 1.531 1.487 1.470 1.463 1.461 1.458 1.457 k 7.1-04 2.6E-04 < 1E-4 < 1E-4 < 1E-4 < 1E-4 < 1E-4 < 1E-4 The resulting optical properties of the thin film are dependent on process conditions like deposition rate and substrate temperature. SiO 2 thin films exhibit an amorphous structure. High packing densities can be achieved by using IAD or a high substrate temperature. NOTES FOR EVAPORATION Evaporator source Electron beam evaporator Crucible / Boat Copper crucible Evaporation temperature 1800 – 2200 °C Deposition rate 0.4 – 1.2 nm/s Oxygen partial pressure none to ~5·10 -5 mbar IAD settings (Leybold APS) 130 – 150 V Bias Substrate temperature conventional RT to 350 °C IAD @ RT QCR-settings Density 2.648 g/cm³, z-ratio 1.0 The evaporation characteristics and the properties of the coating depend on the focusing of the electron beam. -

SYNTHESIS of SOME ALUMINIUM SILICATES* by R. M. CARR~ And
SYNTHESIS OF SOME ALUMINIUM SILICATES* By R. M. CARR~and J. B. DIXON~ Studies in the system A12034Si02 under hydrothermal conditions where three different starting materials (amorphous alumina-quartz, amorphous silica-kaolinite, quartz-kaolinite) were investigated in "pinched-tube" experiments1 were followed by a redetermination in sealed-tube experiments of the synthesis fields obtained from amorphous silica-kaolinite and quartz-kaolinite.2 A synthesis diagram for the starting material amorphous alumina-quartz has now been redetermined in sealed-tube experiments. Experimental The pressure equipment used is similar to that already described.2 Modifications to the equipment, in the form of stellite test-tube bombs each coupled to a Bourdon pressure gauge and controlled with an Ether transitrol temperature regulator, enabled more effective control to be attained in some of the experiments. Errors in temperature control were within &l% at all temperatures. Quartz and amorphous (chromatographic) alumina were mixed in the molecular proportions A1203,4SiOz and Aln03,6SiOz. Samples together with water were placed in silver capsules and sealed. Runs commenced with rapid heating periods (e.g. from room temperature to 400' in 20 min) and were terminated with air quenching. Products were examined with a Philips X-ray diffractometer using filtered Cu radiation. Results A comparison of synthesis field boundary temperatures for pinched-tube and sealed-tube experiments with the starting material amorphous alumina-quartz is given in Table 1. Minor amounts of boehmite were present in many low-temperature runs, indicating sluggish reaction between this phase and quartz. Two decomposition experiments provided confirmation of the synthesis boundaries. Synthetic corundum heated at 490' under a hydrostatic pressure of 30000 lb/inZ was partly altered to pyrophyllite, and kaolinite was obtained from synthetic pyrophyllite at 370". -

Nature of Salt Nasa Summer of Innovation
National Aeronautics and Space Administration NATURE OF SALT NASA SUMMER OF INNOVATION LESSON DESCRIPTION UNIT Students examine the molecular Physical Science – States of Matter structure of salt and the elements that compose it. GRADE LEVELS 4th -6th OBJECTIVES Students will: CONNECTION TO CURRICULUM Explain the general relationship Science between an element's Periodic Table Group Number and its TEACHER PREPARATION TIME 10 minutes tendency to gain or lose electron(s). LESSON TIME NEEDED Explain the difference between 45 minute Complexity: Basic molecular compounds and ionic compounds. Use a model to demonstrate sodium chloride's cubic form which results from its microscopic crystal lattice. Describe the nature of the electrostatic attraction of the oppositely charged ions that holds the structure of salt together. NATIONAL STANDARDS National Science Education Standards (NSTA) Science as Inquiry Understanding of scientific concepts Skills necessary to become independent inquirers about the natural world Physical Science Properties of objects and materials MANAGEMENT It is suggested that the “Electrolysis of Water” activity listed in the additional resources section of this lesson be performed prior to introducing this lesson to students. It can be done as a demonstration or as a full engagement activity with students. CONTENT RESEARCH Chemically, table salt consists of two elements, sodium (Na) and chloride (Cl). Neither element occurs separately and free in nature, but are found bound together as the compound sodium chloride. It occurs naturally in many parts of the world as the mineral halite and as mixed evaporites in salt lakes. Seawater contains an average of 2.6% (by weight) sodium chloride, or 78 million metric tons per cubic kilometer, an inexhaustible supply. -

(Quartz) Silica, Sio2, Is a White Or Colorless Crystalline Compound
Silica (quartz) Silica, SiO2, is a white or colorless crystalline compound found mainly as quartz, sand, flint, and many other minerals. Silica is an important ingredient to manufacture a wide variety of materials. Quartz; Quartz is the most abundant silica mineral. Pure Quartz is colorless and transparent. It occurs in most igneous and practically all metamorphic and sedimentary rocks. It is used as a component of numerous industrial materials. Silicon (Si) has the atomic number 14 and is closely related to carbon. It is a relatively inert metalloid. Silicon is often used for microchips, glass, cement, and pottery. Silica is the most abundant mineral found in the crust of the earth. One of the most common uses of silica quarts is the manufacturer of glass. Silica is the fourteenth element on the periodic table. It can sometimes be found as the substance, quartz which is usually used in jewelry, test tubes, and when placed under pressure, generates an electrical charge. Quartz is the second most abundant mineral in the Earth's crust. It is a clear, glossy mineral with a hardness of 7 on the MOHS scale. Silica, Sa,is a component of glass and concrete. A form of Silica commonly known as quartz, Silica tetrahedra, is the second most common mineral in the earth's crust, it comes in many different forms. Silica is a compound of silicon and oxygen. Earth's outer crust contains 59% of this material. It has three major rock forms, which are quartz, tridymite, and cristobalite. Silica, commonly known in the form of quartz, is the dioxide form of silicon, SiO2.