Cancer Testis Antigen Promotes Triple Negative Breast Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alpha Actinin 4: an Intergral Component of Transcriptional
ALPHA ACTININ 4: AN INTERGRAL COMPONENT OF TRANSCRIPTIONAL PROGRAM REGULATED BY NUCLEAR HORMONE RECEPTORS By SIMRAN KHURANA Submitted in partial fulfillment of the requirements for the degree of doctor of philosophy Thesis Advisor: Dr. Hung-Ying Kao Department of Biochemistry CASE WESTERN RESERVE UNIVERSITY August, 2011 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of SIMRAN KHURANA ______________________________________________________ PhD candidate for the ________________________________degree *. Dr. David Samols (signed)_______________________________________________ (chair of the committee) Dr. Hung-Ying Kao ________________________________________________ Dr. Edward Stavnezer ________________________________________________ Dr. Leslie Bruggeman ________________________________________________ Dr. Colleen Croniger ________________________________________________ ________________________________________________ May 2011 (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. TABLE OF CONTENTS LIST OF TABLES vii LIST OF FIGURES viii ACKNOWLEDEMENTS xii LIST OF ABBREVIATIONS xiii ABSTRACT 1 CHAPTER 1: INTRODUCTION Family of Nuclear Receptors 3 Mechanism of transcriptional regulation by co-repressors and co-activators 8 Importance of LXXLL motif of co-activators in NR mediated transcription 12 Cyclic recruitment of co-regulators on the target promoters 15 Actin and actin related proteins (ABPs) in transcription -

List of Genes Associated with Sudden Cardiac Death (Scdgseta) Gene
List of genes associated with sudden cardiac death (SCDgseta) mRNA expression in normal human heart Entrez_I Gene symbol Gene name Uniprot ID Uniprot name fromb D GTEx BioGPS SAGE c d e ATP-binding cassette subfamily B ABCB1 P08183 MDR1_HUMAN 5243 √ √ member 1 ATP-binding cassette subfamily C ABCC9 O60706 ABCC9_HUMAN 10060 √ √ member 9 ACE Angiotensin I–converting enzyme P12821 ACE_HUMAN 1636 √ √ ACE2 Angiotensin I–converting enzyme 2 Q9BYF1 ACE2_HUMAN 59272 √ √ Acetylcholinesterase (Cartwright ACHE P22303 ACES_HUMAN 43 √ √ blood group) ACTC1 Actin, alpha, cardiac muscle 1 P68032 ACTC_HUMAN 70 √ √ ACTN2 Actinin alpha 2 P35609 ACTN2_HUMAN 88 √ √ √ ACTN4 Actinin alpha 4 O43707 ACTN4_HUMAN 81 √ √ √ ADRA2B Adrenoceptor alpha 2B P18089 ADA2B_HUMAN 151 √ √ AGT Angiotensinogen P01019 ANGT_HUMAN 183 √ √ √ AGTR1 Angiotensin II receptor type 1 P30556 AGTR1_HUMAN 185 √ √ AGTR2 Angiotensin II receptor type 2 P50052 AGTR2_HUMAN 186 √ √ AKAP9 A-kinase anchoring protein 9 Q99996 AKAP9_HUMAN 10142 √ √ √ ANK2/ANKB/ANKYRI Ankyrin 2 Q01484 ANK2_HUMAN 287 √ √ √ N B ANKRD1 Ankyrin repeat domain 1 Q15327 ANKR1_HUMAN 27063 √ √ √ ANKRD9 Ankyrin repeat domain 9 Q96BM1 ANKR9_HUMAN 122416 √ √ ARHGAP24 Rho GTPase–activating protein 24 Q8N264 RHG24_HUMAN 83478 √ √ ATPase Na+/K+–transporting ATP1B1 P05026 AT1B1_HUMAN 481 √ √ √ subunit beta 1 ATPase sarcoplasmic/endoplasmic ATP2A2 P16615 AT2A2_HUMAN 488 √ √ √ reticulum Ca2+ transporting 2 AZIN1 Antizyme inhibitor 1 O14977 AZIN1_HUMAN 51582 √ √ √ UDP-GlcNAc: betaGal B3GNT7 beta-1,3-N-acetylglucosaminyltransfe Q8NFL0 -

The Endocytic Membrane Trafficking Pathway Plays a Major Role
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Liverpool Repository RESEARCH ARTICLE The Endocytic Membrane Trafficking Pathway Plays a Major Role in the Risk of Parkinson’s Disease Sara Bandres-Ciga, PhD,1,2 Sara Saez-Atienzar, PhD,3 Luis Bonet-Ponce, PhD,4 Kimberley Billingsley, MSc,1,5,6 Dan Vitale, MSc,7 Cornelis Blauwendraat, PhD,1 Jesse Raphael Gibbs, PhD,7 Lasse Pihlstrøm, MD, PhD,8 Ziv Gan-Or, MD, PhD,9,10 The International Parkinson’s Disease Genomics Consortium (IPDGC), Mark R. Cookson, PhD,4 Mike A. Nalls, PhD,1,11 and Andrew B. Singleton, PhD1* 1Molecular Genetics Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 2Instituto de Investigación Biosanitaria de Granada (ibs.GRANADA), Granada, Spain 3Transgenics Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 4Cell Biology and Gene Expression Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 5Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom 6Department of Pathophysiology, University of Tartu, Tartu, Estonia 7Computational Biology Group, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 8Department of Neurology, Oslo University Hospital, Oslo, Norway 9Department of Neurology and Neurosurgery, Department of Human Genetics, McGill University, Montréal, Quebec, Canada 10Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montréal, Quebec, Canada 11Data Tecnica International, Glen Echo, Maryland, USA ABSTRACT studies, summary-data based Mendelian randomization Background: PD is a complex polygenic disorder. -

Evolution of the DAN Gene Family in Vertebrates
bioRxiv preprint doi: https://doi.org/10.1101/794404; this version posted June 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. RESEARCH ARTICLE Evolution of the DAN gene family in vertebrates Juan C. Opazo1,2,3, Federico G. Hoffmann4,5, Kattina Zavala1, Scott V. Edwards6 1Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile. 2David Rockefeller Center for Latin American Studies, Harvard University, Cambridge, MA 02138, USA. 3Millennium Nucleus of Ion Channels-Associated Diseases (MiNICAD). 4 Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology, Mississippi State University, Mississippi State, 39762, USA. Cite as: Opazo JC, Hoffmann FG, 5 Zavala K, Edwards SV (2020) Institute for Genomics, Biocomputing, and Biotechnology, Mississippi State Evolution of the DAN gene family in University, Mississippi State, 39762, USA. vertebrates. bioRxiv, 794404, ver. 3 peer-reviewed and recommended by 6 PCI Evolutionary Biology. doi: Department of Organismic and Evolutionary Biology, Harvard University, 10.1101/794404 Cambridge, MA 02138, USA. This article has been peer-reviewed and recommended by Peer Community in Evolutionary Biology Posted: 29 June 2020 doi: 10.24072/pci.evolbiol.100104 ABSTRACT Recommender: Kateryna Makova The DAN gene family (DAN, Differential screening-selected gene Aberrant in Neuroblastoma) is a group of genes that is expressed during development and plays fundamental roles in limb bud formation and digitation, kidney formation and morphogenesis and left-right axis specification. -

Multivariate Analysis Reveals Differentially Expressed Genes
www.nature.com/scientificreports OPEN Multivariate analysis reveals diferentially expressed genes among distinct subtypes of difuse astrocytic gliomas: diagnostic implications Nerea González‑García1,2, Ana Belén Nieto‑Librero1,2, Ana Luisa Vital3, Herminio José Tao4, María González‑Tablas2,5,6, Álvaro Otero2, Purifcación Galindo‑Villardón1,2, Alberto Orfao2,5,6 & María Dolores Tabernero2,5,6,7* Diagnosis and classifcation of gliomas mostly relies on histopathology and a few genetic markers. Here we interrogated microarray gene expression profles (GEP) of 268 difuse astrocytic gliomas—33 difuse astrocytomas (DA), 52 anaplastic astrocytomas (AA) and 183 primary glioblastoma (GBM)—based on multivariate analysis, to identify discriminatory GEP that might support precise histopathological tumor stratifcation, particularly among inconclusive cases with II–III grade diagnosed, which have diferent prognosis and treatment strategies. Microarrays based GEP was analyzed on 155 difuse astrocytic gliomas (discovery cohort) and validated in another 113 tumors (validation set) via sequential univariate analysis (pairwise comparison) for discriminatory gene selection, followed by nonnegative matrix factorization and canonical biplot for identifcation of discriminatory GEP among the distinct histological tumor subtypes. GEP data analysis identifed a set of 27 genes capable of diferentiating among distinct subtypes of gliomas that might support current histological classifcation. DA + AA showed similar molecular profles with only a few discriminatory genes -

Rabbit Anti-ADAM9/FITC Conjugated Antibody-SL20104R-FITC
SunLong Biotech Co.,LTD Tel: 0086-571- 56623320 Fax:0086-571- 56623318 E-mail:[email protected] www.sunlongbiotech.com Rabbit Anti-ADAM9/FITC Conjugated antibody SL20104R-FITC Product Name: Anti-ADAM9/FITC Chinese Name: FITC标记的去整合素样金属蛋白酶9抗体 A disintegrin and metalloproteinase domain 9; A disintegrin and metalloproteinase domain 9; ADAM 9 antibody ADAM metallopeptidase domain 9; Cellular disintegrin Alias: related protein; Disintegrin and metalloproteinase domain 9; MCMP; MDC9; Meltrin gamma; Metalloprotease disintegrin cysteine rich protein 9; Mltng; Myeloma cell metalloproteinase; ADAM9_HUMAN. Organism Species: Rabbit Clonality: Polyclonal React Species: Human,Mouse,Rat,Dog,Cow,Rabbit,Sheep, ICC=1:50-200IF=1:50-200 Applications: not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. Molecular weight: 88kDa Form: Lyophilized or Liquid Concentration: 2mg/1ml immunogen: KLH conjugated synthetic peptide derived from human ADAM9 Lsotype: IgGwww.sunlongbiotech.com Purification: affinity purified by Protein A Storage Buffer: 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. The lyophilized antibody is stable at room temperature for at least one month and for greater than a year Storage: when kept at -20°C. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. background: This gene encodes a member of the ADAM (a disintegrin and metalloprotease domain) family. Members of this family are membrane-anchored proteins structurally related to Product Detail: snake venom disintegrins, and have been implicated in a variety of biological processes involving cell-cell and cell-matrix interactions, including fertilization, muscle development, and neurogenesis. -

Identification of Differentially Expressed Genes Between Primary Lung Cancer and Lymph Node Metastasis Via Bioinformatic Analysis
3754 ONCOLOGY LETTERS 18: 3754-3768, 2019 Identification of differentially expressed genes between primary lung cancer and lymph node metastasis via bioinformatic analysis NAN ZHANG and SHAO-WEI ZHANG Department of Thoracic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050000, P.R. China Received January 10, 2019; Accepted July 12, 2019 DOI: 10.3892/ol.2019.10723 Abstract. Lung cancer (LC), with its high morbidity and cBioPortal. A total of 308 DEGs were identified in the two mortality rates, is one of the most widespread and malignant microarray datasets, which were mainly enriched during neoplasms. Mediastinal lymph node metastasis (MLNM) cellular processes, including the Gene Ontology terms ‘cell’, severely affects postoperative survival of patients with LC. ‘catalytic activity’, ‘molecular function regulator’, ‘signal trans- Additionally, the molecular mechanisms of LC with MLNM ducer activity’ and ‘binding’. The PPI network was composed (MM LC) remain not well understood. To identify the key of 315 edges and 167 nodes. Its significant module had 11 hub biomarkers in its carcinogenesis and development, the data- genes, and high expression of actin β, MYC, arginine vaso- sets GSE23822 and GSE13213 were obtained from the Gene pressin, vesicle associated membrane protein 2 and integrin Expression Omnibus database. The differentially expressed subunit β1, and low expression of NOTCH1, synaptojanin 2 genes (DEGs) were identified, and the Database for Annotation, and intersectin 2 were significantly associated with poor OS. Visualization and Integrated Discovery was used to perform In summary, hub genes and DEGs presented in the present functional annotations of DEGs. Search Tool for the Retrieval study may help identify underlying targets for diagnostic and of Interacting Genes and Cytoscape were utilized to obtain the therapeutic methods for MM LC. -

Alpha;-Actinin-4 Promotes Metastasis in Gastric Cancer
Laboratory Investigation (2017) 97, 1084–1094 © 2017 USCAP, Inc All rights reserved 0023-6837/17 α-Actinin-4 promotes metastasis in gastric cancer Xin Liu and Kent-Man Chu Metastasis increases the mortality rate of gastric cancer, which is the third leading cause of cancer-associated deaths worldwide. This study aims to identify the genes promoting metastasis of gastric cancer (GC). A human cell motility PCR array was used to analyze a pair of tumor and non-tumor tissue samples from a patient with stage IV GC (T3N3M1). Expression of the dysregulated genes was then evaluated in GC tissue samples (n = 10) and cell lines (n =6) via qPCR. Expression of α-actinin-4 (ACTN4) was validated in a larger sample size (n = 47) by qPCR, western blot and immunohistochemistry. Knockdown of ACTN4 with specific siRNAs was performed in GC cells, and adhesion assays, transwell invasion assays and migration assays were used to evaluate the function of these cells. Expression of potential targets of ACTN4 were then evaluated by qPCR. Thirty upregulated genes (greater than twofold) were revealed by the PCR array. We focused on ACTN4 because it was upregulated in 6 out of 10 pairs of tissue samples and 5 out of 6 GC cell lines. Further study indicated that ACTN4 was upregulated in 22/32 pairs of tissue samples at stage III & IV (P = 0.0069). Knockdown of ACTN4 in GC cells showed no significant effect on cell proliferation, but significantly increased cell-matrix adhesion, as well as reduced migration and invasion of AGS, MKN7 and NCI-N87 cells. -
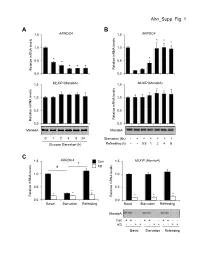
Ahn Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0
Ahn_Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0 * * 0.5 * 0.5 * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 1.5 MLXIP (MondoA) 1.5 MLXIP (MondoA) 1.0 1.0 0.5 0.5 Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 MondoA MondoA 0124824 Starvation (6h) -++++++ Glucose Starvation (h) Refeeding (h) --0.51248 C 1.5 ARRDC4 1.5 MLXIP (MondoA) † Con # KD 1.0 1.0 0.5 0.5 * * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative * * 0.0 0.0 BasalStarvation Refeeding BasalStarvation Refeeding MondoA Con + + - - + + - - + + - - KD - - + + - - + + - - + + BasalStarvation Refeeding Supplemental Figure 1. Glucose-mediated regulation of ARRDC4 is dependent on MondoA in human skeletal myotubes. (A) (top) ARRDC4 and MLXIP (MondoA) mRNA levels were determined by qRT-PCR in human skeletal myotubes following deprivation of glucose at the indicated time (n=4). (bottom) Representative Western blot analysis of MondoA demonstrating the effect of glucose deprivation. *p<0.05 vs. 0h. (B) (top) ARRDC4 and MLXIP (MondoA) expression in human myotubes following a 6h glucose removal and refeeding at the times indicated (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs Starvation 6h. (C) (top) Expression of ARRDC4 and MLXIP in human myotubes following deprivation and refeeding of glucose in the absence or presence of siRNA-mediated MondoA KD (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs siControl. # p<0.05. § p<0.05. The data represents mean ± SD. All statistical significance determined by one-way ANOVA with Tukey multiple comparison post-hoc test. -

Proteomic Expression Profile in Human Temporomandibular Joint
diagnostics Article Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction Andrea Duarte Doetzer 1,*, Roberto Hirochi Herai 1 , Marília Afonso Rabelo Buzalaf 2 and Paula Cristina Trevilatto 1 1 Graduate Program in Health Sciences, School of Medicine, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba 80215-901, Brazil; [email protected] (R.H.H.); [email protected] (P.C.T.) 2 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru 17012-901, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-41-991-864-747 Abstract: Temporomandibular joint dysfunction (TMD) is a multifactorial condition that impairs human’s health and quality of life. Its etiology is still a challenge due to its complex development and the great number of different conditions it comprises. One of the most common forms of TMD is anterior disc displacement without reduction (DDWoR) and other TMDs with distinct origins are condylar hyperplasia (CH) and mandibular dislocation (MD). Thus, the aim of this study is to identify the protein expression profile of synovial fluid and the temporomandibular joint disc of patients diagnosed with DDWoR, CH and MD. Synovial fluid and a fraction of the temporomandibular joint disc were collected from nine patients diagnosed with DDWoR (n = 3), CH (n = 4) and MD (n = 2). Samples were subjected to label-free nLC-MS/MS for proteomic data extraction, and then bioinformatics analysis were conducted for protein identification and functional annotation. The three Citation: Doetzer, A.D.; Herai, R.H.; TMD conditions showed different protein expression profiles, and novel proteins were identified Buzalaf, M.A.R.; Trevilatto, P.C. -

Integrating Protein Copy Numbers with Interaction Networks to Quantify Stoichiometry in Mammalian Endocytosis
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.29.361196; this version posted October 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Integrating protein copy numbers with interaction networks to quantify stoichiometry in mammalian endocytosis Daisy Duan1, Meretta Hanson1, David O. Holland2, Margaret E Johnson1* 1TC Jenkins Department of Biophysics, Johns Hopkins University, 3400 N Charles St, Baltimore, MD 21218. 2NIH, Bethesda, MD, 20892. *Corresponding Author: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.10.29.361196; this version posted October 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Abstract Proteins that drive processes like clathrin-mediated endocytosis (CME) are expressed at various copy numbers within a cell, from hundreds (e.g. auxilin) to millions (e.g. clathrin). Between cell types with identical genomes, copy numbers further vary significantly both in absolute and relative abundance. These variations contain essential information about each protein’s function, but how significant are these variations and how can they be quantified to infer useful functional behavior? Here, we address this by quantifying the stoichiometry of proteins involved in the CME network. We find robust trends across three cell types in proteins that are sub- vs super-stoichiometric in terms of protein function, network topology (e.g. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in