Development and Developmental Disorders of the Brain Stem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience
CNs 5, 7, 9, 10 - Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience PHARYNGEAL ARCH SET, CNS 5, 7, 9, 10 • They are derived from the pharyngeal (aka branchial) arches • They have special motor and autonomic motor functions CRANIAL NERVES EXIT FROM THE BRAINSTEM CN 5, the trigeminal nerve exits the mid/lower pons.* CN 7, the facial nerve exits the pontomedullary junction.* CN 9, the glossopharyngeal nerve exits the lateral medulla.* CN 10, the vagus nerve exits the lateral medulla.* CRANIAL NERVE NUCLEI AT BRAINSTEM LEVELS Midbrain • The motor trigeminal nucleus of CN 5. Nerve Path: • The motor division of the trigeminal nerve passes laterally to enter cerebellopontine angle cistern. Pons • The facial nucleus of CN 7. • The superior salivatory nucleus of CN 7. Nerve Path: • CN 7 sweeps over the abducens nucleus as it exits the brainstem laterally in an internal genu, which generates a small bump in the floor of the fourth ventricle: the facial colliculus • Fibers emanate from the superior salivatory nucleus, as well. Medulla • The dorsal motor nucleus of the vagus, CN 10 • The inferior salivatory nucleus, CN 9 1 / 3 • The nucleus ambiguus, CNs 9 and 10. Nerve Paths: • CNs 9 and 10 exit the medulla laterally through the post-olivary sulcus to enter the cerebellomedullary cistern. THE TRIGEMINAL NERVE, CN 5  • The motor division of the trigeminal nerve innervates the muscles of mastication • It passes ventrolaterally through the cerebellopontine angle cistern and exits through foramen ovale as part of the mandibular division (CN 5[3]). Clinical Correlation - Trigeminal Neuropathy THE FACIAL NERVE, CN 7  • The facial nucleus innervates the muscles of facial expression • It spans from the lower pons to the pontomedullary junction. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Anatomy of the Brainstem
Anatomy of the Brainstem Neuroanatomy block-Anatomy-Lecture 5 Editing file Objectives At the end of the lecture, students should be able to: 01 List the components of brain stem. 02 Describe the site of brain stem 03 Describe the relations between components of brain stem & their relations to cerebellum. 04 Describe the external features of both ventral & dorsal surfaces of brain stem Color guide 05 List cranial nerves emerging from brain stem 06 Describe the site of emergence of each cranial nerve ● Only in boys slides in Green ● Only in girls slides in Purple ● important in Red ● Notes in Grey Development of Brain Brain stem ● The brain develops from the cranial part of neural tube. ● The brainstem is the region of the brain that connects the ● The cranial part is divided into 3 parts: cerebrum with the spinal cord. ● Site: It lies on the basilar part of occipital bone (clivus). - Subdivided into: ● Parts from above downwards : 1. Telencephalon: (cavities: 2 lateral ventricles) 1. Midbrain Two cerebral hemispheres. Forebrain 2. Pons 2. Diencephalon: (cavity: 3rd ventricle) 3. Medulla oblongata thalamus, hypothalamus, epithalamus & subthalamus ● Connection with cerebellum: Each part of the brain stem is connected to the Midbrain - (cavity: cerebral aqueduct) cerebellum by cerebellar peduncles (superior, middle & inferior). - (cavity: 4th ventricle) - Subdivided into: Hindbrain 1. Pons 2. Cerebellum 3. Medulla oblongata 3 Sagittal section of Brain 4 Functions of the Brain Stem Pathway of tracts between cerebral cortex & spinal cord (ascending and descending tracts). 1 Site of origin of nuclei of cranial nerves (from 3rd to 12th). 2 Site of emergence of cranial nerves (from 3rd to 12th). -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3. -

Morphometric Assesment of the External Anatomy of Fourth Ventricle and Dorsal Brainstem in Fresh Cadavers
DOI: 10.5137/1019-5149.JTN.24942-18.1 Turk Neurosurg 29(3):445-450, 2019 Received: 26.09.2018 / Accepted: 20.11.2018 Published Online: 19.12.2018 Original Investigation Morphometric Assesment of the External Anatomy of Fourth Ventricle and Dorsal Brainstem in Fresh Cadavers Veysel ANTAR1, Okan TURK1, Salim KATAR2, Mahmut OZDEN3, Balkan SAHIN4, Sahin YUCELI5, Erdogan KARA6, Ayse YURTSEVEN6 1Istanbul Training and Research Hospital, Department of Neurosurgery, Istanbul, Turkey 2Selahattin Eyyubi City Hospital, Department of Neurosurgery, Diyarbakir, Turkey 3Bahcesehir University, Department of Neurosurgery, Istanbul, Turkey 4Sultan Abdulhamit Han Training and Research Hospital, Department of Neurosurgery, Istanbul, Turkey 5Erzincan Neon Hospital, Department of Neurosurgery, Erzincan, Turkey 6Ministry of Justice, Council of Forensic Medicine, Istanbul, Turkey Corresponding author: Veysel ANTAR [email protected] ABSTRACT AIM: To investigate the external anatomy of the fourth ventricle and dorsal brainstem using morphometric data, which could be useful for preoperative surgical planning. MATERIAL and METHODS: Between January 2017 and December 2017, 42 fresh adult cadavers were investigated for the measurements of the cadaver brainstems and fourth ventricle, and they were recorded by photography. Measurements were evaluated according to body mass indexes (BMIs) of the patients. We also investigate the visualization of facial colliculus and stria medullaris on brainstem. RESULTS: A total of 42 fresh cadavers with a mean age of 45.38 ± 16.41 years old were included in this research. We found no statistically significant difference between measurements and BMIs. Facial colliculus was visualized in 92.9% (n=39), but it could not visualized in 7.1% (n=3) of the subjects. -

Seventh-And-A-Half Syndrome
Ophthalmology And Ophthalmic Surgery Open Access Case Report Seventh-and-a-Half Syndrome Ama Sadaka*, Shauna Berry and Andrew G Lee Department of Ophthalmology, Blanton Eye Institute, the Methodist Hospital, USA A R T I C L E I N F O A B S T R A C T Article history: Received: 04 September 2017 This is a case of a patient with right internuclear ophthalmoplegia and right Accepted: 05 October 2017 Published: 13 October 2017 peripheral seventh nerve palsy with no other neurologic deficits. Magnetic Keywords: resonance imaging showed a small localized right hemipons infarct involving Seven-and-a-half syndrome; INO facial motor nucleus and facial genu as well as the right medial longitudinal fasciculus. We introduce “Seven-and-a-half syndrome” as a new Copyright: ©2017 Sadaka A clinicoradiologic syndrome. Ophthalmol Ophthalmic Surg This is an open access article distributed Case Presentation under the Creative Commons Attribution License, which permits unrestricted use, A 68-year-old white female presented with sudden onset horizontal binocular distribution, and reproduction in any medium, provided the original work is diplopia and right-sided facial weakness involving the upper and lower face properly cited. consistent with a severe lower motor neuron seventh nerve palsy. Past medical Citation this article: Sadaka A, Berry S, Lee AG. Seventh-and-a-Half Syndrome. history was significant for uncontrolled hypertension and cerebral amyloid Ophthalmol Ophthalmic Surg. 2017; 1(1):112. angiopathy. No history of head trauma or infection. Visual acuity was 20/20 in both eyes. Pupils were equal and reactive with no relative afferent pupillary defect in either eye. -

Inter-And Intrapatient Variability of Facial Nerve Response Areas in The
http://www.ncbi.nlm.nih.gov/pubmed/21206320. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. University of Zurich http://www.zora.uzh.ch Zurich Open Repository and Archive Originally published at: Bertalanffy, H; Tissira, N; Krayenbühl, N; Bozinov, O; Sarnthein , J (2011). Inter- and intrapatient variability of facial nerve response areas in the floor of the fourth ventricle. Neurosurgery, 68(1 Supp):23-31. Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2011 Inter- and intrapatient variability of facial nerve response areas in the floor of the fourth ventricle Bertalanffy, H; Tissira, N; Krayenbühl, N; Bozinov, O; Sarnthein , J http://www.ncbi.nlm.nih.gov/pubmed/21206320. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: Bertalanffy, H; Tissira, N; Krayenbühl, N; Bozinov, O; Sarnthein , J (2011). Inter- and intrapatient variability of facial nerve response areas in the floor of the fourth ventricle. Neurosurgery, 68(1 Supp):23-31. Inter- and intrapatient variability of facial nerve response areas in the floor of the fourth ventricle Abstract BACKGROUND: Surgical exposure of intrinsic brainstem lesions through the floor of the 4th ventricle requires precise identification of facial nerve (CN VII) fibers to avoid damage. OBJECTIVE: To assess the shape, size, and variability of the area where the facial nerve can be stimulated electrophysiologically on the surface of the rhomboid fossa. METHODS: Over a period of 18 months, 20 patients were operated on for various brainstem and/or cerebellar lesions. -
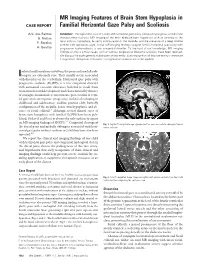
MR Imaging Features of Brain Stem Hypoplasia in Familial Horizontal
MR Imaging Features of Brain Stem Hypoplasia in CASE REPORT Familial Horizontal Gaze Palsy and Scoliosis A.V. dos Santos SUMMARY: We report the case of a child with horizontal gaze palsy, pendular nystagmus, and discrete S. Matias thoracolumbar scoliosis. MR imaging of the brain depicted pons hypoplasia with an absence of the facial colliculi, hypoplasia, butterfly configuration of the medulla, and the presence of a deep midline P. Saraiva pontine cleft (split pons sign). These MR imaging findings suggest familial horizontal gaze palsy with A. Goula˜o progressive kyphoscoliosis, a rare congenital disorder. To the best of our knowledge, MR imaging findings of only 4 similar cases, with or without progressive idiopathic scoliosis, have been reported. We discuss the pathogenesis substratum of this entity. Early recognition of this rare entity is important if supportive therapeutic measures in progressive scoliosis are to be applied. solated malformations involving the pons and medulla ob- Ilongata are extremely rare. They usually occur associated with disorders of the cerebellum. Horizontal gaze palsy with progressive scoliosis (HGPPS) is a rare congenital disorder with autosomal recessive inherence, believed to result from cranial nuclear maldevelopment and characterized by absence of conjugate horizontal eye movements, preservation of verti- cal gaze and convergence, progressive scoliosis developing in childhood and adolescence, midline pontine cleft, butterfly configuration of the medulla, brain stem hypoplasia, and ab- sence of facial colliculi.1 Although several clinical cases of brain stem hypoplasia with familial HGPPS have been pub- lished, Pieh et al and Rossi et al were the only authors to report on MR imaging findings of HGPPS.2,3 Congenital cleavage of Fig 1. -

Differentiation of the Bulbar Motor Nuclei and the Coincident Develop- Ment of Associated Root Fibers in the Rsbbit
DIFFERENTIATION OF THE BULBAR MOTOR NUCLEI AND THE COINCIDENT DEVELOP- MENT OF ASSOCIATED ROOT FIBERS IN THE RSBBIT DONALD L. KIMMEL Department of Anatomy, University of Michigan,’ Aim Arbor THIRTY-ONE FIGURES CONTENTS Introduction ........................................................ 83 Material and methods ................................................ 84 General survey of the literature ....................................... 85 Description of material studied ........................................ 86 Somatic efferent component. Its central and peripheral development .... 86 The hypoglossal ............................................. 86 The abdueens ............................................... 96 Visceral efferent component. Its central and peripheral development .... 103 The vago-accessory ........................................... 103 The glossopharyngeal ........................................ 124 The facial .................................................. 129 The trigemiiial .............................................. 137 Geiicrxldisrussion .................................................... 143 INTRODUCTION This present study concerns itself primarily with the onto- genetic development of the nuclear centers of bulbar cranial nerves in the rabbit and with the embryonic and adult distri- butions of their branches. Its purpose is to show that the central development proceeds stage for stage with the pro- A dissertation submitted in partial fulfillment of the requirements for the degree of doctor of philosophy -

Brainstem: Structure & Func On
Brainstem: Structure & Func1on Adrian Poniatowski StudyAid Neuroanatomy Seminar November 26, 2017 A pleasure to meet you... • Name: Adrian Poniatowski • Hometown: New York City • Job: Professional Baller, Future Doctor • Hobbies: Winning @ Life Brainstem = Manha<an • Connects everything • Every connecon is important • Small lesions = big problems • Locaon, locaon, locaon! Ques1on 1 Cranial nerves exit from the following locaons EXCEPT • a.) vagus nerve from posterior lateral sulcus • b.) facial nerve from cerebello-ponne angle • c.) abducens nerve from anterior lateral sulcus • d.) trochlear nerve lateral to the frenulum of the superior medullary velum • e.) oculomotor nerve from interpeduncular fossa 3 Midbrain Pons Cross-secons: Clinical Correlates Medulla Ques1on 2 All of the following locaons given are correct EXCEPT: • a.) motor nucleus of trigeminal - ponne tegmentum • b.) superior salivatory nucleus of VII - ponne tegmentum • c.) superior vesMbular nucleus of VIII - ponne tegmentum • d.) motor nucleus of hypoglossal - dorsal medulla • e.) motor nucleus of VI - mesencephalic tegmentum MLF Pathway • FuncMon: Connects oculomotor nuclei to integrate eye movements • Nerves: CN III and VI • Lesion causes Internuclear Opthalmoplegia • Abnormal adducMon of IPSILATERAL eye, usually with nystagmus • Where is the lesion here? Ques1on 3 Muscles of the eyeball are innervated by all of the following EXCEPT: • a.) axons of the nucleus located in the mesencephalic tegmentum • b.) axons of the nucleus located in the ponne tegmentum • c.) axons -

MR Imaging of Brain-Stem Hypoplasia in Horizontal Gaze Palsy with Progressive Scoliosis
AJNR Am J Neuroradiol 25:1046–1048, June/July 2004 Case Report MR Imaging of Brain-Stem Hypoplasia in Horizontal Gaze Palsy with Progressive Scoliosis Andrea Rossi, Martin Catala, Roberta Biancheri, Raffaella Di Comite, and Paolo Tortori-Donati Summary: We present the MR imaging findings of a girl MR imaging of the entire neuraxis at our institution before with horizontal gaze palsy and progressive scoliosis spinal surgery (Fig 1). Imaging findings were normal except for (HGPPS). HGPPS is a rare congenital disorder character- double-curve thoracolumbar scoliosis. Brain MR imaging re- ized by absence of conjugate horizontal eye movements and vealed a hypoplastic pons in which the posterior two-thirds were split into two halves by a midsagittal cleft extending accompanied by progressive scoliosis developing in child- ventrally from the fourth ventricular floor, generating a split hood and adolescence. MR imaging depicted brain-stem pons sign on axial images. The facial colliculi were absent, and hypoplasia with absence of the facial colliculi, presence of a the fourth ventricular floor was tent shaped. The medulla was deep midline pontine cleft (split pons sign), and a butterfly also hypoplastic and showed a butterfly configuration. The configuration of the medulla. These MR imaging features inferior olivary nuclei were prominent with respect to the suggest the diagnosis of HGPPS and correlate with the pyramids, and the prominence of the gracile and cuneate nuclei on the posterior aspect of the medulla was absent. clinical findings. We hypothesize that maldevelopment of dorsomedial brain-stem structures plays a crucial role in the pathogenesis of HGPPS. Discussion The first descriptions of associated horizontal gaze Horizontal gaze palsy with progressive scoliosis palsy and scoliosis date back to 30 years. -

Neuroanatomy, Cranial Nerve 6 (Abducens) - Statpearls - NCBI Bookshelf
12/17/2018 Neuroanatomy, Cranial Nerve 6 (Abducens) - StatPearls - NCBI Bookshelf NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Neuroanatomy, Cranial Nerve 6 (Abducens) Authors Van Nguyen; Matthew Varacallo1. Affilations 1 Department of Orthopaedic Surgery, University of Kentucky School of Medicine Last Update: October 27, 2018. Introduction Cranial nerve six (CN VI), also known as the abducens nerve, is one of the nerves responsible for the extraocular motor functions of the eye, along with the oculomotor nerve (CN III) and the trochlear nerve (CN IV). Structure and Function Unlike the oculomotor nerve and the trochlear nerve, the abducens nerve is a purely somatic nerve, meaning the nerve has no sensory function. Its main function is to carry general somatic efferent nerve axons to innervate the lateral rectus muscle which then abducts the eye on the ipsilateral side. It is also secondarily involved in innervation of the contralateral medial rectus muscle by way of the medial longitudinal fasciculus so that both eyes move laterally in a coordinated manner. Nerves The nerve itself can be divided into four distinct portions: the nucleus, the cisternal portion, the cavernous sinus portion, and the orbital portion. The abducens nucleus resides in the dorsal pons, ventral to the floor of the fourth ventricle, and just lateral to the medial longitudinal fasciculus. About forty percent of the axons project through the ipsilateral medial longitudinal fasciculus to cross over to the contralateral medial rectus subnucleus to eventually innervate the contralateral medial rectus muscle.