Topography and Cytoarchitecture of the Motor Nuclei in the Brainstem of Salamanders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience
CNs 5, 7, 9, 10 - Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience PHARYNGEAL ARCH SET, CNS 5, 7, 9, 10 • They are derived from the pharyngeal (aka branchial) arches • They have special motor and autonomic motor functions CRANIAL NERVES EXIT FROM THE BRAINSTEM CN 5, the trigeminal nerve exits the mid/lower pons.* CN 7, the facial nerve exits the pontomedullary junction.* CN 9, the glossopharyngeal nerve exits the lateral medulla.* CN 10, the vagus nerve exits the lateral medulla.* CRANIAL NERVE NUCLEI AT BRAINSTEM LEVELS Midbrain • The motor trigeminal nucleus of CN 5. Nerve Path: • The motor division of the trigeminal nerve passes laterally to enter cerebellopontine angle cistern. Pons • The facial nucleus of CN 7. • The superior salivatory nucleus of CN 7. Nerve Path: • CN 7 sweeps over the abducens nucleus as it exits the brainstem laterally in an internal genu, which generates a small bump in the floor of the fourth ventricle: the facial colliculus • Fibers emanate from the superior salivatory nucleus, as well. Medulla • The dorsal motor nucleus of the vagus, CN 10 • The inferior salivatory nucleus, CN 9 1 / 3 • The nucleus ambiguus, CNs 9 and 10. Nerve Paths: • CNs 9 and 10 exit the medulla laterally through the post-olivary sulcus to enter the cerebellomedullary cistern. THE TRIGEMINAL NERVE, CN 5  • The motor division of the trigeminal nerve innervates the muscles of mastication • It passes ventrolaterally through the cerebellopontine angle cistern and exits through foramen ovale as part of the mandibular division (CN 5[3]). Clinical Correlation - Trigeminal Neuropathy THE FACIAL NERVE, CN 7  • The facial nucleus innervates the muscles of facial expression • It spans from the lower pons to the pontomedullary junction. -

Projections from the Trigeminal Nuclear Complex to the Cochlear Nuclei: a Retrograde and Anterograde Tracing Study in the Guinea Pig
Journal of Neuroscience Research 78:901–907 (2004) Projections From the Trigeminal Nuclear Complex to the Cochlear Nuclei: a Retrograde and Anterograde Tracing Study in the Guinea Pig Jianxun Zhou and Susan Shore* Department of Otolaryngology and Kresge Hearing Research Institute, University of Michigan, Ann Arbor, Michigan In addition to input from auditory centers, the cochlear cuneate nucleus innervation of cochlear nucleus has been nucleus (CN) receives inputs from nonauditory centers, hypothesized to convey information about head and pinna including the trigeminal sensory complex. The detailed position for the purpose of localizing a sound source in anatomy, however, and the functional implications of the space (Young et al., 1995). In addition, interactions be- nonauditory innervation of the auditory system are not tween somatosensory and auditory systems have been fully understood. We demonstrated previously that the linked increasingly to phantom sound perception, also trigeminal ganglion projects to CN, with terminal labeling known as tinnitus. This is demonstrated in the observa- most dense in the marginal cell area and secondarily in tions that injuries of the head and neck region can lead to the magnocellular area of the ventral cochlear nucleus the onset of tinnitus in patients with no hearing loss (VCN). We continue this line of study by investigating the (Lockwood et al., 1998). projection from the spinal trigeminal nucleus to CN in We demonstrated previously projections from the guinea pig. After injections of the retrograde tracers Flu- trigeminal ganglion to CN in guinea pigs (Shore et al., oroGold or biotinylated dextran amine (BDA) in VCN, 2000). Terminal labeling of trigeminal ganglion projec- labeled cells were found in the spinal trigeminal nuclei, tions to the CN was found to be most dense in the most densely in the pars interpolaris and pars caudalis marginal cell area and secondarily in the magnocellular with ipsilateral dominance. -

Circuits in the Rodent Brainstem That Control Whisking in Concert with Other Orofacial Motor Actions
Neuroscience 368 (2018) 152–170 CIRCUITS IN THE RODENT BRAINSTEM THAT CONTROL WHISKING IN CONCERT WITH OTHER OROFACIAL MOTOR ACTIONS y y LAUREN E. MCELVAIN, a BETH FRIEDMAN, a provides the reset to the relevant premotor oscillators. HARVEY J. KARTEN, b KAREL SVOBODA, c FAN WANG, d Third, direct feedback from somatosensory trigeminal e a,f,g MARTIN DESCHEˆ NES AND DAVID KLEINFELD * nuclei can rapidly alter motion of the sensors. This feed- a Department of Physics, University of California at San Diego, back is disynaptic and can be tuned by high-level inputs. La Jolla, CA 92093, USA A holistic model for the coordination of orofacial motor actions into behaviors will encompass feedback pathways b Department of Neurosciences, University of California at San Diego School of Medicine, La Jolla, CA 92093, USA through the midbrain and forebrain, as well as hindbrain c areas. Howard Hughes Medical Institute, Janelia Research This article is part of a Special Issue entitled: Barrel Campus, Ashburn, VA 20147, USA Cortex. Ó 2017 IBRO. Published by Elsevier Ltd. All rights d Department of Neurobiology, Duke University Medical reserved. Center, Durham, NC 27710, USA e Department of Psychiatry and Neuroscience, Laval University, Que´bec City, G1J 2G3, Canada f Key words: coupled oscillators, facial nucleus, hypoglossal Section of Neurobiology, University of California at San Diego, La Jolla, CA 92093, USA nucleus, licking, orienting, tongue, vibrissa. g Department of Electrical and Computer Engineering, University of California at San Diego, La Jolla, CA 92093, USA Contents Introduction 153 Abstract—The world view of rodents is largely determined Coordination of multiple orofacial motor actions 153 by sensation on two length scales. -

DR. Sanaa Alshaarawy
By DR. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal, mid “Trigeminal level” and rostral). 3. Mid brain ( superior and inferior colliculi). Describe the Reticular formation (structure, function and pathway) being an important content of the brain stem. 2 1. Traversed by the Central Canal. Motor Decussation*. Spinal Nucleus of Trigeminal (Trigeminal sensory nucleus)* : ➢ It is a larger sensory T.S of Caudal part of M.O. nucleus. ➢ It is the brain stem continuation of the Substantia Gelatinosa of spinal cord 3 The Nucleus Extends : Through the whole length of the brain stem and upper segments of spinal cord. It lies in all levels of M.O, medial to the spinal tract of the trigeminal. It receives pain and temperature from face, forehead. Its tract present in all levels of M.O. is formed of descending fibers that terminate in the trigeminal nucleus. 4 It is Motor Decussation. Formed by pyramidal fibers, (75-90%) cross to the opposite side They descend in the Decuss- = crossing lateral white column of the spinal cord as the lateral corticospinal tract. The uncrossed fibers form the ventral corticospinal tract. 5 Traversed by Central Canal. Larger size Gracile & Cuneate nuclei, concerned with proprioceptive deep sensations of the body. Axons of Gracile & Cuneate nuclei form the internal arcuate fibers; decussating forming Sensory Decussation. Pyramids are prominent ventrally. 6 Formed by the crossed internal arcuate fibers Medial Leminiscus: Composed of the ascending internal arcuate fibers after their crossing. -

University International
INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photo graphed the photographer has followed a definite method in “sectioning” the material. It is customary to begin filming at the upper left hand corner of a large sheet and to continue from left to right in equal sections with small overlaps. If necescary, sectioning is continued again—beginning below the first row and continuing on until complete. 4. For any illustrations that cannot be reproduced satisfactorily by xerography, photographic prints can be purchased at additional cost and tipped into your xerographic copy. -

Cortical Control of Facial Expression
Review The Journal of Comparative Neurology Research in Systems Neuroscience DOI 10.1002/cne.23908 Topical review Cortical control of facial expression. René M. Müri1,2,3 1Division of Cognitive and Restorative Neurology, Departments of Neurology and Clinical Research, University Hospital Inselspital, Bern, Switzerland 2Gerontechnology and Rehabilitation Group, University of Bern, Bern, Switzerland 3Center for Cognition, Learning, and Memory, University of Bern, Bern, Switzerland Abbreviated title: Cortical control of facial expression Key words: human, facial expression, emotion, facial innervation | downloaded: 13.3.2017 http://boris.unibe.ch/72088/ This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as an ‘Accepted Article’, doi: 10.1002/cne.23908 source: © 2015 Wiley Periodicals, Inc. Received: Jan 31, 2015; Revised: Sep 19, 2015; Accepted: Sep 25, 2015 This article is protected by copyright. All rights reserved. Journal of Comparative Neurology Page 4 of 23 Abstract The present topical review deals with the motor control of facial expressions in humans. Facial expressions are a central part of human communication. Emotional face expressions have a crucial role in human non-verbal behavior, allowing a rapid transfer of information between individuals. Facial expressions can be both voluntarily or emotionally controlled. Recent studies in non-human primates and humans revealed that the motor control of facial expressions has a distributed neural representation. At least 5 cortical regions on the medial and lateral aspects of each hemisphere are involved: the primary motor cortex, the ventral lateral premotor cortex, the supplementary motor area on the medial wall, and, finally, the rostral and caudal cingulate cortex. -

Somatotopic Organization of Perioral Musculature Innervation Within the Pig Facial Motor Nucleus
Original Paper Brain Behav Evol 2005;66:22–34 Received: September 20, 2004 Returned for revision: November 10, 2004 DOI: 10.1159/000085045 Accepted after revision: December 7, 2004 Published online: April 8, 2005 Somatotopic Organization of Perioral Musculature Innervation within the Pig Facial Motor Nucleus Christopher D. Marshall a Ron H. Hsu b Susan W. Herring c aTexas A&M University at Galveston, Galveston, Tex., bDepartment of Pediatric Dentistry, University of North Carolina, Chapel Hill, N.C., and cDepartment of Orthodontics, University of Washington, Seattle, Wash., USA Key Words pools of the lateral 4 of the 7 subnuclei of the facial motor Somatotopy W Innervation W Facial nucleus W Perioral nucleus. The motor neuron pools of the perioral muscles muscles W Orbicularis oris W Buccinator W Mammals were generally segregated from motoneurons innervat- ing other facial muscles of the rostrum. However, motor neuron pools were not confined to single nuclei but Abstract instead spanned across 3–4 subnuclei. Perioral muscle The orbicularis oris and buccinator muscles of mammals motor neuron pools overlapped but were organized so- form an important subset of the facial musculature, the matotopically. Motor neuron pools of portions of the perioral muscles. In many taxa, these muscles form a SOO overlapped greatly with each other but exhibited a robust muscular hydrostat capable of highly manipula- crude somatotopy within the SOO motor neuron pool. tive fine motor movements, likely accompanied by a spe- The large and somatotopically organized SOO motor cialized pattern of innervation. We conducted a retro- neuron pool in pigs suggests that the upper lip might be grade nerve-tracing study of cranial nerve (CN) VII in pigs more richly innervated than the other perioral muscles (Sus scrofa) to: (1) map the motor neuron pool distribu- and functionally divided. -

Monosynaptic Premotor Circuit Tracing Reveals Neural Substrates for Oro-Motor Coordination
1 Title 2 3 Monosynaptic Premotor Circuit Tracing Reveals Neural Substrates 4 for Oro-motor Coordination 5 6 7 8 9 10 11 Authors and affiliations 12 13 Edward Stanek IV1, Steven Cheng1, Jun Takatoh1, Bao-Xia Han1, and Fan Wang1, 2 * 14 15 1. Department of Neurobiology, Duke University Medical Center, Durham, NC 27710. 16 2. Department of Cell Biology, Duke University Medical Center, Durham, NC 27710. 17 18 19 20 21 22 Corresponding author 23 24 *Fan Wang 25 Department of Neurobiology, Box 3209 26 Duke University Medical Center 27 Durham, NC 27710 28 Phone: 919-684-3682 29 e-mail: [email protected] 30 31 Number of pages: 59 32 Number of Figures: 10 33 Number of Movies: 4 34 Number of Tables: 2 35 Number of words, Abstract: 237 36 Number of words, Main Text: 5,168 1 37 ABSTRACT 38 39 Feeding behaviors require intricately coordinated activation among the muscles of the jaw, 40 tongue, and face, but the neural anatomical substrates underlying such coordination remain 41 unclear. Here we investigate whether the premotor circuitry of jaw and tongue motoneurons 42 contain elements for coordination. Using a modified monosynaptic rabies virus based 43 transsynaptic tracing strategy, we systematically mapped premotor neurons for the jaw-closing 44 masseter muscle and the tongue-protruding genioglossus muscle. The maps revealed that the two 45 groups of premotor neurons are distributed in regions implicated in rhythmogenesis, descending 46 motor control, and sensory feedback. Importantly, we discovered several premotor connection 47 configurations that are ideally suited for coordinating bilaterally symmetric jaw movements, and 48 for enabling co-activation of specific jaw, tongue, and facial muscles. -

Lecture (6) Internal Structures of the Brainstem.Pdf
Internal structures of the Brainstem Neuroanatomy block-Anatomy-Lecture 6 Editing file Objectives At the end of the lecture, students should be able to: ● Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal and rostral). 3. Midbrain ( superior and inferior colliculi). Color guide ● Only in boys slides in Green ● Only in girls slides in Purple ● important in Red ● Notes in Grey Medulla oblongata Caudal (Closed) Medulla Traversed by the central canal Motor decussation (decussation of the pyramids) ● Formed by pyramidal fibers, (75-90%) cross to the opposite side ● They descend in the lateral white column of the spinal cord as the lateral corticospinal tract. ● The uncrossed fibers form the ventral corticospinal tract Trigeminal sensory nucleus. ● it is the larger sensory nucleus. ● The Nucleus Extends Through the whole length of the brainstem and its note :All CN V afferent sensory information enters continuation of the substantia gelatinosa of the spinal cord. the brainstem through the nerve itself located in the pons. Thus, to reach the spinal nucleus (which ● It lies in all levels of M.O, medial to the spinal tract of the trigeminal. spans the entire brain stem length) in the Caudal ● It receives pain and temperature from face, forehead. Medulla those fibers have to "descend" in what's known as the Spinal Tract of the Trigeminal ● Its tract present in all levels of M.O. is formed of descending (how its sensory and descend?see the note) fibers that terminate in the trigeminal nucleus. -

Experimental Determination of the Primary Trigeminal Projections in Lampreys
Brain Research, 163 (1979) 323-327 323 © Elsevier/North-Holland Biomedical Press Experimental determination of the primary trigeminal projections in lampreys R. GLENN NORTHCUTT Division of Biological Sciences, The University of Michigan, Ann Arbor, Mich. 48109 (U.S.A.) (Accepted November 9th, 1978) The trigeminal complex of lampreys, like that of anamniotic gnathostomes, consists of profundus and 'mandibulo 'mandibulomaxillary' branchesa, s. The pro- fundus branch possesses a distinct ganglion (supraorbital ganglion) separate from the ganglion (suboptical ganglion) of the 'mandibulomaxillary' brancheslL However, both ganglia send their processes into the medulla as a single trigeminal sensory root (Figs. 1A, 2A). The lamprey trigeminal complex differs from that of other anamniotes in that the anterior lateral line and facial ganglia are not closely associated with the trigeminal ganglia but are located beneath and within the optic capsule a,12. This separation clearly simplifies experimental analysis of the trigeminal projections, as the trigeminal ganglia can be lesioned or the sensory roots transected without damaging facial or lateralis inputs. Earlier studies claim that scattered lateral line receptors on the dorsal surface of the head are innervated by the trigeminal nerve~,~. This condition has not been reported for any other vertebrate and has been interpreted as supporting the hypothesis that a lateralis component existed ancestrally in each branchiomeric cranial nerve. It is also claimed that the trigeminal nerve in lampreys projects directly to the cerebellum, the octavolateralis area and the funicular nucleus (presumed precursor of the dorsal column nuclei4,6). This trigeminal projection pattern has been interpreted as supporting the claim that the dorsal column nuclei, octavolaterlis area and cerebellum arose from a single somatic sensory column, and that lampreys essentially retain that primitive condition6. -

Cranial Nerves
Cranial Nerves Cranial nerve evaluation is an important part of a neurologic exam. There are some differences in the assessment of cranial nerves with different species, but the general principles are the same. You should know the names and basic functions of the 12 pairs of cranial nerves. This PowerPage reviews the cranial nerves and basic brain anatomy which may be seen on the VTNE. The 12 Cranial Nerves: CN I – Olfactory Nerve • Mediates the sense of smell, observed when the pet sniffs around its environment CN II – Optic Nerve Carries visual signals from retina to occipital lobe of brain, observed as the pet tracks an object with its eyes. It also causes pupil constriction. The Menace response is the waving of the hand at the dog’s eye to see if it blinks (this nerve provides the vision; the blink is due to cranial nerve VII) CN III – Oculomotor Nerve • Provides motor to most of the extraocular muscles (dorsal, ventral, and medial rectus) and for pupil constriction o Observing pupillary constriction in PLR CN IV – Trochlear Nerve • Provides motor function to the dorsal oblique extraocular muscle and rolls globe medially © 2018 VetTechPrep.com • All rights reserved. 1 Cranial Nerves CN V – Trigeminal Nerve – Maxillary, Mandibular, and Ophthalmic Branches • Provides motor to muscles of mastication (chewing muscles) and sensory to eyelids, cornea, tongue, nasal mucosa and mouth. CN VI- Abducens Nerve • Provides motor function to the lateral rectus extraocular muscle and retractor bulbi • Examined by touching the globe and observing for retraction (also tests V for sensory) Responsible for physiologic nystagmus when turning head (also involves III, IV, and VIII) CN VII – Facial Nerve • Provides motor to muscles of facial expression (eyelids, ears, lips) and sensory to medial pinna (ear flap). -
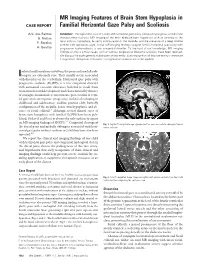
MR Imaging Features of Brain Stem Hypoplasia in Familial Horizontal
MR Imaging Features of Brain Stem Hypoplasia in CASE REPORT Familial Horizontal Gaze Palsy and Scoliosis A.V. dos Santos SUMMARY: We report the case of a child with horizontal gaze palsy, pendular nystagmus, and discrete S. Matias thoracolumbar scoliosis. MR imaging of the brain depicted pons hypoplasia with an absence of the facial colliculi, hypoplasia, butterfly configuration of the medulla, and the presence of a deep midline P. Saraiva pontine cleft (split pons sign). These MR imaging findings suggest familial horizontal gaze palsy with A. Goula˜o progressive kyphoscoliosis, a rare congenital disorder. To the best of our knowledge, MR imaging findings of only 4 similar cases, with or without progressive idiopathic scoliosis, have been reported. We discuss the pathogenesis substratum of this entity. Early recognition of this rare entity is important if supportive therapeutic measures in progressive scoliosis are to be applied. solated malformations involving the pons and medulla ob- Ilongata are extremely rare. They usually occur associated with disorders of the cerebellum. Horizontal gaze palsy with progressive scoliosis (HGPPS) is a rare congenital disorder with autosomal recessive inherence, believed to result from cranial nuclear maldevelopment and characterized by absence of conjugate horizontal eye movements, preservation of verti- cal gaze and convergence, progressive scoliosis developing in childhood and adolescence, midline pontine cleft, butterfly configuration of the medulla, brain stem hypoplasia, and ab- sence of facial colliculi.1 Although several clinical cases of brain stem hypoplasia with familial HGPPS have been pub- lished, Pieh et al and Rossi et al were the only authors to report on MR imaging findings of HGPPS.2,3 Congenital cleavage of Fig 1.