Fungal Systematics and Evolution PAGES 97–170
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

French Guiana, and Stamps International, Krakow
1 provisional overprints as old stocks were used up for different purposes from the FRENCH FRENCH ones originally intended. GUIANA GUIANA In 1946 Guyane and Inini were combined together as an Overseas Department of The French first established their presence FISCAL France. Some revenues of France continued in 1763. A Penal Colony was set up in 1854 to be overprinted GUYANE for use there, at St Laurent in the Province of Maroni. (GENERAL and several unoverprinted values of French Another penal camp on Devils Island off sets were only released in Guyane. the coast (which became nororious during DUTY) the Dreyfus Affair) gave the colony a bad Recently a satellite launching base was reputation. established in Guyane and economic activity has increased. The administrative centre was at Cayenne, and settlements for logging and for farming Thanks to the following collectors for tropical produce such as coffee, sugar, information and scans : cocoa, manioc and (most important of all) Andrew McClellan, sugar cane spread along the coast. Initially, Before 1934, regular "Fiscal" issues of Tony Hall, revenues of France were used when required France were used in French Guiana, and Stamps International, Krakow. on documents, and may be recognised only can only be distinguished by the cancel. _______________________________________ by the cancellation. 1934. Timbre Fiscal stamps of France of 1925, Type Daussy, ovpt GUYANE. Typo. No wmk. Perf 13½. 1. 1c red-brown, brown & blue ........... 2.50 A cancel "GF" in a roller of dots was used 2. 2c red-brown, brown & blue ........... 2.50 in Guiana. 3. 3c red-brown, brown & blue .......... -

In the Land of Forgotten Colors on the Trail of Cranach, Dürer and Stradivari: Chemist Georg Kremer Studies and Reproduces the Paints Used by the Old Masters
PRINTING INKS AND COLORS In the land of forgotten colors On the trail of Cranach, Dürer and Stradivari: chemist Georg Kremer studies and reproduces the paints used by the old masters. 34 print process 22/03 TEXT: HELGE BENDL, PHOTOS: KURT HENSELER magician’s wares: dragon’s blood, their brushes in pigments; artists, archi- While Kremer’s wife stokes up the dried cuttlefish ink, red fermented tects and even violin-makers today prefer ceramic woodstove in the living room (lab A rice, charred ivory, curcuma pow- these rediscovered natural materials to workers later process the soot from the der, gallnuts, buckthorn bark and walnut mass-produced industrial paints. beechwood fire into bistre, an historical shells, plus bismuth, ink stone, Russian In the mid-Eighties, Kremer purchased pigment previously used to copy bibles), jade, Spanish ocher, rock crystal and dried the old mill at the southwestern tip of Georg Kremer describes his never-end- cochineal insects, as well as an extract from Bavaria. Today, the company and its mul- ing search for historical formulations, for a purple dye murex that costs 2,000 euros ti-million sales support not only Kremer’s natural substances old and new: “Just as a gram. The most valuable substances are family, but also some thirty employees. words have gone out of use in the course of more precious than gold. An alchemist in Although shops have been opened in time, pigments have also been forgotten. the heart of Bavaria, inhabiting an historic Munich, Stuttgart and New York, Kremer Lukas Cranach even had his own apothe- mill in the village of Aichstetten? continues to coordinate his worldwide cary and sold a variety of substances, mix- Georg Kremer laughs at the idea. -

COOLWALL® Colors
CLEAR TO MISTY COLORS White Lavender 1A-2P Whisper Pink 3A-2P Pink Hibiscus 4A-2P Feminique 5A-2P Sunset Snow 6A-2P Peach Frosting 15A-2P Burgundy Dash 7A-1A Pink Frost 7A-2P Cosmetic Pink 14A-2P Lover’s Knot 14A-3P Santa Fe 14B-3D Mineral Red 14B-4D September Cream 17A-2P Invanhoe Ivory 16A-2P Peach Dust 15A-3P Pleasant Pebble 16A-3P Peach Beige 18A-2P Peach Whisper 17A-3P Abbeystone 18A-3P Candelabra 19A-3P Orange Scent 19A-2P Orange Paste 18B-1P Frothy Orange 18B-2T Orange Drop 18B-3D Apricot Liquer 18C-1P Colonial Peach 18C-2T Mango 18C-3D Lacquer Orange 18C-4D Banana Split 27A-2P Candleglow 19B-1P Orange Gem 19B-2T Tangerine 19B-3D Moon Morn 19C-1P Gobi Sands 19C-2T Squash 19C-3D Indian Corn 19C-4D Patio Brick 20A-1A Vanilla Ice 20A-2P Powder Puff 20A-3P Honey Rose 20B-1P Calfskin 20B-2T Smoked Seville 20B-3D Cattail 20B-4D Vicuna 20C-1P Italian Earth 20C-2T Copper Kettle 20C-3D Hash Brown 20C-4D Abigail 27C-1T Golden Corn 27C-2T Clarion 27C-3D These sample reproductions are an approximation of color and do not denote the actual texture or sheen and vary according to material type and specific batch. Colors also vary depending upon lighting conditions, substrate, exposure, and on-line viewing. On-line viewing is for reference purposes and is not intended for final color choice; it is recommended to refer to Tex-Cote's physical fan deck for final color selection. -

Postage Stamp Collectors Curios, Coins and Kindred Sciences
5 fgs A D V A N C E COPY m Vol. I.. No. I The September, 1908 Canadian. Collector ■ Wt .'1 m m m DEVOTED TO THE INTERESTS OF Postage Stamp Collectors ■ Curios, Coins 1 $ and Kindred Sciences. & m f t S M I f : m TO SUBSCRIBERS As a Special Offer to new subscribers we offer for this month : 25 Canadian, including old jubilees, old map stamps, old 8c. K ing’s Head, 5c. and 7c., etc., and 5 Quebec Tercen tenary stamps, including #c. and 5c. values - $0 So1 ALL Packet of 50 differeut stam ps - - - - - 75 FOR 20 word exchange advt. ------- 20 Subscription to The Canadiaq Collector for one year - - 50 50c. $2.25 Canadian and American subscribers please send cash or money orders. Eng lish, Colonial, and all others send International Money Orders, or unused English or Canadian low value stamps, no others accepted. TO ADVERTISERS SPECIAL .—-ALL our pages are 8 1 /2 x 1112 inches, which is nearly twice as large as other collectors’ papers, considering this our rates are very reasonable. There will be 3000 extra copies of the October issue sent out, that is, 3000 copiesi besides the number we send out every month. This means that it will reach thousands of collectors* in every country where the English lan guage is spoken. Every advertiser knows that the next six months are THE months for advertising, and you will find our rates exceptionally reasonable consider ing our circulation, etc. The October issue will contain at least twenty-four pages of interesting matter. -

Precancels/Canada
PRECANCELS/ CANADA WHOLE NUMBER 1 • SPRING, 2000 GEOFF WALBURN 1902-1999 PRECANCELLED Henry Geoffrey Walburn was born in Bramhall, Cheshire, ADMIRAL England on September 21, 1902. He died in Kelowna, SHADES British Columbia on by Hans Reiche September 4, 1999, at age 96. Geoff was predeceased by The question that was raised his wife Bernice (nee by A. Ellwood about the lack Delaney) in 1995. of data of the Admiral shades that were precancelled During Geoff's lifetime, he originated from this writer. sold real estate, worked on Admirals are full of shades and farms, owned and operated partially listed in the Unitrade a large apple orchard, and, catalogue. prior to his retirement, owned and operated a purposes to be used and For example the 4 cents exists summer resort named Pixie enjoyed by advanced and in olive bistre, olive yellow, Beach which overlooks novice collectors throughout golden yellow and yellow Okanagan Lake just north of the stamp collecting ochre. The golden yellow is the the Okanagan Centre post community. best and often undervalued. office. During much of the Geoff was a founding member The 5 cents exists in dark blue, above, he was ably assisted of the Okanagan Mainline grey blue and indigo. The 5 by his wife Bernice. Philatelic Association which cents violet shades are not as Geoff was an accomplished recently celebrated its 40th pronounced. bridge player and belonged anniversary. He belonged to The 7 cents has some very nice to many clubs over the years. numerous stamp fraternities shades such as the straw, sage An avid reader, he was which included BNAPS, CPS of green, greenish yellow and constantly busy at a number GB, RPSC, PHS of Canada, and olive bistre. -

OIL PAINTING GLOSSARY Alkyd (Pronounced: Al-Kid)
OIL PAINTING GLOSSARY Alkyd (Pronounced: al-kid) An alkyd is a synthetic resin that can be added to oil paint to speed up the drying time of oil paints. You can buy an alkyd-based medium that you can mix in with your oils; the most commonly available is Liquin by Winsor & Newton. “Alla Prima” (Pronounced: ah-luh pree-ma) Alla Prima is an Italian oil painting technique, usually from life, in which the entire painting is completed in one session or while the paint is still wet. Usually, there isn’t any underpainting to the piece. Portraits, landscapes, and still life are common subject matter using alla prima. It translates as “at the first”. In past eras, it was used primarily as a means of sketching, but eventually, it became a means of producing finished works of art by the Impressionists. “Bistre” Bistre (“the wipe-out method”) is an underpainting using warm browns (usually raw umber or burnt umber). A thin wash of Raw umber is painted over the white canvas and then ‘wiped out’ to create a tonal underpainting. The shadows are built up using thin color, allowing the warmth of the brown to show through while the lights and midtones are applied as opaque color. You can also use Burnt Umber for an even warmer, darker underpainting. The Bistre method lends itself very well to chiaroscuro. Chiaroscuro (Pronounced: key-ARE-oh-SCURE-oh) Chiaroscuro is an Italian word literally meaning “light dark”, used to describe the skillful balance of light and dark in a painting with strong contrasts to create a dramatic effect. -

Woodgrain Prints
woodgrain prints The beginning of something stunning. Custom Color Matching Durable Woodgrain Panels No High Order Minimums Fabrication to Exact Specifications Eco-friendly Paint Process Cut-to-Size and Custom Fabricated woodgrain prints by Panel Processing, Inc. The Details • 4' x 8' sheets stocked in 3.2mm & 5.5mm Panel Processing’s Woodgrain Prints are a different type of panel. It’s not • UV Top Coated digitally printed, it’s not veneer and it’s not laminate. The realistic look of Panel’s • Meets All KCMA Standards Woodgrain Prints is achieved utilizing a gravure printing method with a one to • Printed One Side, Two Sided Available three color process that goes directly onto the substrate. This direct transfer • Custom Color Matching Available print application offers a consistent transfer of ink and a high quality image with • Cut-to-Size repeatable color and consistency. Multiple engraved cylinders of different wood • Custom Fabricated species give this collection of Woodgrain Prints diversity and character for multiple • Perforated Upon Request projects. Although custom colors are available, the standard colors listed below • Meets All KCMA Standards are complementary to many industry laminates. Start your next project with Panel Processing's Woodgrain Prints...the beginning of something stunning. The Standard Colors PPI-001 PPI-002 PPI-003 PPI-004 Cocoa Bean Java Acer Maple Autumn Mist PPI-005 PPI-006 PPI-007 PPI-008 Cognac Flax Maple Bistre Brown Bourbon PPI-009 PPI-010 PPI-011 PPI-012 Holland Oak Zebrano Burnt Umber Hilltop Cherry PPI-013 PPI-014 Montgomery Maple Panel Mahogany 120 North Industrial Hwy. -

C L E a R T O M I S T Y C O L O
CLEAR TO MISTY COLORS White Lavender 1A-2P Whisper Pink 3A-2P Pink Hibiscus 4A-2P Feminique 5A-2P Sunset Snow 6A-2P Peach Frosting 15A-2P Burgundy Dash 7A-1A Pink Frost 7A-2P Cosmetic Pink 14A-2P Lover’s Knot 14A-3P Santa Fe 14B-3D Mineral Red 14B-4D September Cream 17A-2P Invanhoe Ivory 16A-2P Peach Dust 15A-3P Pleasant Pebble 16A-3P Peach Beige 18A-2P Peach Whisper 17A-3P Abbeystone 18A-3P Candelabra 19A-3P Orange Scent 19A-2P Orange Paste 18B-1P Frothy Orange 18B-2T Orange Drop 18B-3D Apricot Liquer 18C-1P Colonial Peach 18C-2T Mango 18C-3D Lacquer Orange 18C-4D Banana Split 27A-2P Candleglow 19B-1P Orange Gem 19B-2T Tangerine 19B-3D Moon Morn 19C-1P Gobi Sands 19C-2T Squash 19C-3D Indian Corn 19C-4D Patio Brick 20A-1A Vanilla Ice 20A-2P Powder Puff 20A-3P Honey Rose 20B-1P Calfskin 20B-2T Smoked Seville 20B-3D Cattail 20B-4D Vicuna 20C-1P Italian Earth 20C-2T Copper Kettle 20C-3D Hash Brown 20C-4D Abigail 27C-1T Golden Corn 27C-2T Clarion 27C-3D These sample reproductions are an approximation of color and do not denote the actual texture or sheen and vary according to material type and specific batch. Colors also vary depending upon lighting conditions, substrate and exposure. When color matching is critical, an applied sample is required and all materials should be from the same batch. Special colors are subject to confirmation by Textured Coatings of America, Inc. -

The General Issues of United States Stamps, Their Shades and Varieties
: \7niUd States Stamps ^^^^^^^^^^^^^^P -ma ^^^mM vmM • : ;.: :v .', . ;':: :... ,..:'v '/',.' :;: .-' ";' :: EY GIBBONS, Inc. 6tl Utsisit, JU«s*»i» 6 as CORNELL UNIVERSITY LIBRARY THIS BOOK IS ONE OF A COLLECTION MADE BY BENNO LOEWY 1854-1919 AND BEQUEATHED TO CORNELL UNIVERSITY THE GENERAL ISSUES OF UNITED STATES STAMPS THEIR SHADES AND VARIETIES TO WHICH IS AFFIXED A HISTORY OF THE PRIVATE PERFORATING MACHINES AND THEIR PRODUCTS BY EUSTACE B. POWER WITH ILLUSTRATIONS 1909 Copyrighted, 1909, by Stanley Gibbons, Inc. FIRST EDITION NEW YORK STANLEY GIBBONS, Incorporated 198 BROADWAY LONDON STANLEY GIBBONS, Limited 391 STRAND r ~~fe~~~& -£-£-~„ c f\l(*(?y l PRESS OF The Hann & Adair Printing Co. columbus, ohio FOREWORD In offering this work to the collector of United States Stamps I do so with the full knowledge that there are more exhaustive works already published, and I do not claim any originality in the work. It has been produced in response to many requests for a guide to shades, and also as a kind of warning to collectors what to avoid and what not to avoid. In the present day the value of unused stamps is often so stupendous that unscrupulous people are tempted to turn a dishonest penny by cleaning pen- struck copies, perforating and gumming proofs, erasing the word "specimen," etc., and it is to warn collectors of these practices that I have at times gone into seemingly minute descriptions. I am exceedingly indebted to the Scott Stamp & Coin Co., Mr. C. H. Mekeel, Mr. John N. Luff and many others for use of portions of their copyright works. -

Pollen Color Chart
Pollen Color Common name Latin name raisin [3] Red Horse chestnut Aesculus carnea caledonian brown White Clover Trifolium repens auburn [3] White Sweet Clover Melilotus alba auburn [3] Yellow Sweet Clover Melilotus officinalis dark brown Crimson Clover Trifolium incarnatum grey brown Red Maple Acer rubrum anatolia [3] Horse chestnut Aesculus hippocastanum orange red, red, purplish Henbit Lamium amplexicaule red orange, red Snowdrop Galanthus nivalis orange Marigold Calendula officinalis red yellow Pear Pyrus communis red yellow, orange Blueberry Vaccínium myrtíllus red yellow, orange Dandelion Taraxacum officinale bright orange Asparagus Asparagus officinalis light brown to brown Cherry Plum Prunus cerasifera canary yellow [3] Elder Sambucus canadensis brownish yellow Grey Alder Alnus incana reddish yellow Peach Prunus persica dark yellow Sour Cherry Prunus cerasus reddish yellow Aster Aster spp. yellow to dark yellow White Sweet Clover Melilotus alba yellow to dark yellow Yellow Sweet Clover Melilotus officinalis yellow brown Sainfoin Onobrychis viciifolia yellow brown Alsike Clover Trifolium hybridum yellow brown Hawthorn Crataegus spp. yellow to light orange Basswood or American Linden Tilia americana dull yellow or black? Ivy Hedera spp. golden Sunflower Helianthus annuus golden Goldenrod Solidago spp. Crocus vernus (syn. Crocus orange yellow Yellow Crocus aureus) khaki [3] Alfalfa Medicago sativa light brown to brown pollen - not considered a good pollen source but Almond Prunus amygdalus bees are the primary pollinator yellow brown, -
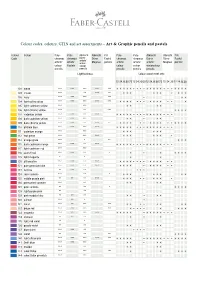
Colour Table
Colour codes, colours, GTIN and set assortments – Art & Graphic pencils and pastels Colour Colour Poly- Poly- Albrecht Albrecht Pitt Poly - Poly - Albrecht Albrecht Pitt Code chromos chromos Dürer Dürer Pastel chromos chromos Dürer Dürer Pastel artists’ artists’ artists’ water- Magnus pencils artists’ artists’ artists’ Magnus pencils colour Pastels colour colour colour water colour pencils pencils pencils pencils pencils Lightfastness Colour assortment sets 12 24 3660 7212 24 36 6012 24 3660 72 12 24 30 12 24 3660 101 white *** *** *** *** *** • • • • • • • • • • • • • • • • • • • • • 102 cream *** ** *** ** • • • • • • • • • • 103 ivory *** *** *** *** • • • 104 light yellow glaze *** *** *** *** *** • • • • • • • • • • • • • • 105 light cadmium yellow *** *** • • • • 106 light chrome yellow *** ** *** • • • • 107 cadmium yellow *** *** ** *** • • • • • • • • • • • • • • • • • 108 dark cadmium yellow *** *** *** • • • • • 109 dark chrome yellow *** *** ** *** ** • • • • • • • • • • • • • • • • 110 phthalo blue *** *** *** *** • • • • • • • • • • • • • • • • 111 cadmium orange *** *** • • • • • • 112 leaf green *** *** *** • • • • • • • 113 orange glaze *** * ** • • • 115 dark cadmium orange *** *** ** *** • • • • • • • • • • • • • • • • • 117 light cadmium red *** *** 118 scarlet red *** *** ** * • • • • • • • 119 light magenta * * • • 120 ultramarine *** *** ** *** • • • • • • • • • • • • • • 121 pale geranium lake *** *** ** *** • • • • • • • • • • • 123 fuchsia ** *** ** • • • 124 rose carmine *** ** * • • • • • • • • 125 middle purple -

Designing the Future
3556_01.qxd:3556_09 10/6/08 1:06 PM Page 1 09 65 00/FOS BuyLine 3556 Designing the future Marmoleum Global 3 is the perfect answer to today’s ecologi- cal concerns. As a product made from natural and renewable raw materials, Marmoleum has always been kind to the envi- ronment. Now, in combination with the fantastic new design possibilities contained within our Global 3 collection, it really is possible to design the future: with ecologically sound floor coverings that offer a high design quotient and which are optimized for economical installation and maintenance. Forbo creates better environments in every sense: in terms of design and architectural vision, environmentally, and with regards to the total cost of ownership. To us, that’s what “designing the future” is all about. Each of Dual Tile’s colors has been carefully selected for its balance new! and ability to enhance different tile combinations with color accents and patterns. Available in tile sizes of 13”x13” and 20”x20”. T2713 calico T2607 white marble T3234 forest ground T3223 emerald T3224 chartreuse T2795 butter T3120 rosato T3216 moraine T2621 dove grey T3221 hyacinth T3125 golden sunset T3075 shell T3232 horse roan T2629 eiger T3053 dove blue T3055 fresco blue T3226 marigold T2767 rust T2707 barley T3048 graphite T3220 urban night T3030 blue T3126 kyoto T3228 red amaranth T3233 shitake T3236 dark bistre T2939 black T3218 deep ocean T3127 bleeckerstreet T2784 coffee Forbo Flooring Systems • 1-800-842-7839 • www.forboflooringNA.com • www.floorcostcomparison.com 3556_02.qxd:3556_09 10/6/08 1:08 PM Page 2 Forbo Flooring Systems recently was awarded the Sustainable Materials Rating Technology© (SMaRT©) Sustainable Platinum Certification as a building product for Marmoleum and Bulletin Board Products.