Foothill Sedge (Carex Tumulicola) in Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Draft Plant Propagation Protocol
Plant Propagation Protocol for Carex inops ESRM 412 – Native Plant Production TAXONOMY Family Names Family Scientific Name: Cyperaceae Family Common Name: Sedge Scientific Names Genus: Carex Species: inops Species Authority: L. H. Bailey Variety: Sub-species: Cultivar: Authority for Variety/Sub-species: Common Synonym(s) (include full CAINI3 Carex inops L.H. Bailey ssp inops scientific names (e.g., Elymus CAINH2 Carex inops ssp heliophila (Mack.) Crins glaucus Buckley), including variety Synonyms for ssp heliophila or subspecies information) CAER5 Carex erxlebeniana L. Kelso CAHE5 Carex heliophila Mack. CAPEH Carex pensylvanica Lam. ssp. Heliophila (Mack.) W.A. Weber CAPED Carex pensylvanica Lam. var. digyna Boeckeler Common Name(s): long-stolon sedge or sun sedge (ssp heliophila) Species Code (as per USDA Plants CAIN9 database): GENERAL INFORMATION Geographical range (distribution maps for North America and Washington state) http://plants.usda.gov/java/profile?symbol=CAIN9 http://plants.usda.gov/java/profile?symbol=CAIN9 Ecological distribution (ecosystems it Found in shortgrass, mixed, and tallgrass prairies, as occurs in, etc): well as Ponderosa pine communities and other woodlands (Fryer 2009) Climate and elevation range Dry to seasonally wet climates. Occasionally found at elevations > 5000 ft.(Fryer 2009) Local habitat and abundance; may May dominate to co-dominate in some systems. High include commonly associated prevelance and persistance even in systems where it is species not the dominant species. (Fryer 2009) Plant strategy -

Ecological Site Description Section L: Ecological Site Characteristics Ecological Site Identification and Concept
ESD Printable Report Page 1 of 56 United States Department of Agriculture Natural Resources Conservation Service Ecological Site Description Section l: Ecological Site Characteristics Ecological Site Identification and Concept Site stage: Provisional Provisional: an ESD at the provisional status represents the lowest tier of documentation that is releasable to the public. It contains a grouping of soil units that respond similarly to ecological processes. The ESD contains 1) enough information to distinguish it from similar and associated ecological sites and 2) a draft state and transition model capturing the ecological processes and vegetative states and community phases as they are currently conceptualized. The provisional ESD has undergone both quality control and quality assurance protocols. It is expected that the provisional ESD will continue refinement towards an approved status. Site name: Clayey / Pascopyrum smithii - Nassella viridula ( / western wheatgrass - green needlegrass) Site type: Rangeland Site ID: R058DY011SD Major land resource area (MLRA): 058D-Northern Rolling High Plains, Eastern Part https://esis.sc.egov.usda.gov/ESDReport/fsReportPrt.aspx?id=R058DY011SD&rptLevel=... 5/27/2016 ESD Printable Report Page 2 of 56 Physiographic Features This site occurs on nearly level to moderately steep uplands. Landform: (1) Terrace (2) Hill (3) Plain Minimum Maximum Elevation (feet): 2300 4000 Slope (percent): 0 6 Water table depth (inches): 80 80 Flooding Frequency: None None Ponding Frequency: None None Runoff class: High Very high Aspect: No Influence on this site Climatic Features https://esis.sc.egov.usda.gov/ESDReport/fsReportPrt.aspx?id=R058DY011SD&rptLevel=... 5/27/2016 ESD Printable Report Page 3 of 56 The climate in this MLRA is typical of the drier portions of the Northern Great Plains where sagebrush steppes to the west yield to grassland to the east. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Phytolacca Esculenta Van Houtte
168 CONTENTS BOSABALIDIS ARTEMIOS MICHAEL – Glandular hairs, non-glandular hairs, and essential oils in the winter and summer leaves of the seasonally dimorphic Thymus sibthorpii (Lamiaceae) .................................................................................................. 3 SHARAWY SHERIF MOHAMED – Floral anatomy of Alpinia speciosa and Hedychium coronarium (Zingiberaceae) with particular reference to the nature of labellum and epigynous glands ........................................................................................................... 13 PRAMOD SIVAN, KARUMANCHI SAMBASIVA RAO – Effect of 2,6- dichlorobenzonitrile (DCB) on secondary wall deposition and lignification in the stem of Hibiscus cannabinus L.................................................................................. 25 IFRIM CAMELIA – Contributions to the seeds’ study of some species of the Plantago L. genus ..................................................................................................................................... 35 VENUGOPAL NAGULAN, AHUJA PREETI, LALCHHANHIMI – A unique type of endosperm in Panax wangianus S. C. Sun .................................................................... 45 JAIME A. TEIXEIRA DA SILVA – In vitro rhizogenesis in Papaya (Carica papaya L.) ....... 51 KATHIRESAN KANDASAMY, RAVINDER SINGH CHINNAPPAN – Preliminary conservation effort on Rhizophora annamalayana Kathir., the only endemic mangrove to India, through in vitro method .................................................................................. -
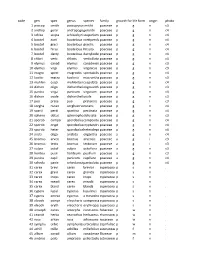
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

– the 2020 Horticulture Guide –
– THE 2020 HORTICULTURE GUIDE – THE 2020 BULB & PLANT MART IS BEING HELD ONLINE ONLY AT WWW.GCHOUSTON.ORG THE DEADLINE FOR ORDERING YOUR FAVORITE BULBS AND SELECTED PLANTS IS OCTOBER 5, 2020 PICK UP YOUR ORDER OCTOBER 16-17 AT SILVER STREET STUDIOS AT SAWYER YARDS, 2000 EDWARDS STREET FRIDAY, OCTOBER 16, 2020 SATURDAY, OCTOBER 17, 2020 9:00am - 5:00pm 9:00am - 2:00pm The 2020 Horticulture Guide was generously underwritten by DEAR FELLOW GARDENERS, I am excited to welcome you to The Garden Club of Houston’s 78th Annual Bulb and Plant Mart. Although this year has thrown many obstacles our way, we feel that the “show must go on.” In response to the COVID-19 situation, this year will look a little different. For the safety of our members and our customers, this year will be an online pre-order only sale. Our mission stays the same: to support our community’s green spaces, and to educate our community in the areas of gardening, horticulture, conservation, and related topics. GCH members serve as volunteers, and our profits from the Bulb Mart are given back to WELCOME the community in support of our mission. In the last fifteen years, we have given back over $3.5 million in grants to the community! The Garden Club of Houston’s first Plant Sale was held in 1942, on the steps of The Museum of Fine Arts, Houston, with plants dug from members’ gardens. Plants propagated from our own members’ yards will be available again this year as well as plants and bulbs sourced from near and far that are unique, interesting, and well suited for area gardens. -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -

Studies on the Genus Carex on the Idaho Panhandle National Forests
STUDIES IN THE GENUS CAREX ON THE IDAHO PANHANDLE NATIONAL FORESTS by Steven L. Caicco Natural Heritage Section Nongame Wildlife/Endangered Species Program Bureau of Wildlife December 1988 Idaho Department of Fish and Game 600 South Walnut Street, P.O. Box 25 Boise, Idaho 83707 Jerry M. Conley, Director Cooperative Challenge Grant Project Idaho Panhandle National Forests Idaho Department of Fish and Game Contract No. 53-0281-7-163 ABSTRACT Eleven of thirteen species of the genus Carex (sedges) which have been designated as Sensitive Species within Region 1 of the U.S. Forest Service are known to occur in Idaho. In this study, the status of these thirteen species, plus one other species of sedge, on the Idaho Panhandle National Forests was investigated through herbaria searches and field surveys. In individual reports, the taxonomy, description, range, habitat, collection record, and conservation status of nine of the fourteen species are discussed. Each species discussion ends with recommendations for land managers and field personnel. The other five species of sedge are more briefly discussed. TABLE OF CONTENTS ABSTRACT...................................................... i TABLE OF CONTENTS............................................. ii LIST OF APPENDICES............................................ ii INTRODUCTION.................................................. 1 INDIVIDUAL SPECIES REPORTS Carex aenea Fern. ............................................ 2 Carex buxbaumii Wahl. ........................................ 4 Carex californica -

Flora of the Stansbury Mountains, Utah
Great Basin Naturalist Volume 43 Number 4 Article 11 10-31-1983 Flora of the Stansbury Mountains, Utah Alan C. Taye U.S. Army Intelligence Center and School, Fort Huachuca, Arizona Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Taye, Alan C. (1983) "Flora of the Stansbury Mountains, Utah," Great Basin Naturalist: Vol. 43 : No. 4 , Article 11. Available at: https://scholarsarchive.byu.edu/gbn/vol43/iss4/11 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. FLORA OF THE STANSBURY MOUNTAINS, UTAH Alan C. Taye' Abstract.— The Stansbury Mountains of north central Utah rise over 2000 m above surrounding desert valleys to a maximum elevation of 3362 m on Deseret Peak. Because of the great variety of environmental conditions that can be found in the Stansburys, a wide range of plant species and vegetation types (from shadscale desert to alpine mead- ow) exist there. This paper presents an annotated list of 594 vascular plant species in 315 genera and 78 families. The largest families are Asteraceae (98 species), Poaceae (71), Brassicaceae (33), Fabaceae (27), and Rosaceae (26). Elymiis flcwescens was previously unreported from Utah. Statistical comparison of the Stansbury flora with neighboring mountain floras indicates that the Wasatch Mountains lying 65 km to the east have probably been the primary source area for development of the Stansbury flora. -

Squilchuck State Park
Rare Plant Inventory and Community Vegetation Survey Squilchuck State Park Cypripedium montanum,mountain lady’s-slipper, on the state Watch list, present at Squilchuck State Park Conducted for The Washington State Pakrs and Recreation Commission PO Box 42650, Olympia, Washington 98504 Conducted by Dana Visalli, Methow Biodiversity Project PO Box 175, Winthrop, WA 98862 In Cooperation with the Pacific Biodiversity Institute December 31, 2004 Rare Plant Inventory and Community Vegetation Survey Squilchuck State Park In the summer of 2004, at the request of and under contract to the Washington State Parks Commission, a rare plant inventory and community vegetation survey was conducted at Squilchuck State Park by Dana Visalli and assisting botanists and GIS technicians. Squilchuck State Park is a 263 acre park on the east slope of the Cascade Mountains in Central Washington, located largely in the transition zone between shrub-steppe and montane forest. Plant community polygons were delineated prior to the initiation of field surveys using or- thophotos and satellite imagery. These polygons were then ground checked during the vegetation surveys, which were conducted simultaneously with the rare plant inventories. All plant associa- tions were determined using theField Guide for Forested Plant Associations of the Wenatchee National Forest(Lilybridge et al, 1995) The Douglas-fir dominated forest above the lodge, on the eastern slopes of the park. The forest on this east slope is in places heavily overstocked and the trees supressed. Vegetation surveys and plant inventories were conducted by two field personnel (one bota- nist, one GIS technician) on June 11th, and again by 4 field workers on August 13 (two botanists and two GIS technicians). -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Vascular Plant Species with Documented Or Recorded Occurrence in Placer County
A PPENDIX II Vascular Plant Species with Documented or Reported Occurrence in Placer County APPENDIX II. Vascular Plant Species with Documented or Reported Occurrence in Placer County Family Scientific Name Common Name FERN AND FERN ALLIES Azollaceae Mosquito fern family Azolla filiculoides Pacific mosquito fern Dennstaedtiaceae Bracken family Pteridium aquilinum var.pubescens Bracken fern Dryopteridaceae Wood fern family Athyrium alpestre var. americanum Alpine lady fern Athyrium filix-femina var. cyclosorum Lady fern Cystopteris fragilis Fragile fern Polystichum imbricans ssp. curtum Cliff sword fern Polystichum imbricans ssp. imbricans Imbricate sword fern Polystichum kruckebergii Kruckeberg’s hollyfern Polystichum lonchitis Northern hollyfern Polystichum munitum Sword fern Equisetaceae Horsetail family Equisetum arvense Common horsetail Equisetum hyemale ssp. affine Scouring rush Equisetum laevigatum Smooth horsetail Isoetaceae Quillwort family Isoetes bolanderi Bolander’s quillwort Isoetes howellii Howell’s quillwort Isoetes orcuttii Orcutt’s quillwort Lycopodiaceae Club-moss family Lycopodiella inundata Bog club-moss Marsileaceae Marsilea family Marsilea vestita ssp. vestita Water clover Pilularia americana American pillwort Ophioglossaceae Adder’s-tongue family Botrychium multifidum Leathery grapefern Polypodiaceae Polypody family Polypodium hesperium Western polypody Pteridaceae Brake family Adiantum aleuticum Five-finger maidenhair Adiantum jordanii Common maidenhair fern Aspidotis densa Indian’s dream Cheilanthes cooperae Cooper’s