Pharmacology & Toxicology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -

OCCASIO I AL PAPER O. 36 RECORDS of the ZOOLOGICAL SURVEY of INDIA
MISCELLANEOUS PUBLICATION OCCASIO I AL PAPER o. 36 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA MISCELLANEOUS PUBLICATION OCCASIONAL PAPER No. 36 A SURVEY OF THE CAUVERY RIVER SYSTEM WITH A MAJOR ACCOUNT OF ITS FISH FAUNA BY K. C. Jayaram Zoological Survey C!! India, Oalcutta-700 016 AND T~ Venkateswarlu" M. B. Ragunathan S.kern Regional Station, Zoological Survey of India, Madras 600 028 Edited by the Director, Zoological Survey. of India 1982 ® Copyright 1982, Government of India Published in August, 1982 PRICE: 1 nlana : Rs. 4~.OO Foreign : £ 6.00 $ 9,50 PRINTED ~N INDIA BY THB BANI PRESS, 16 HBMENDRA SBN STRBBT, CALCUTTA-700 006 AND PUBLISHED BY THB DIRBCTOR, ZOOLOGICAL SURVBY OP INDIA, CALCUTTA. RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA Miscellaneous Publication Occasional Paper No. 36 1982 Pages 1-115 CONTENTS PAGE INTRODUCTION 1 WORK PROGRAMME ... 1 AUTHORSHIP ASSIGNMENTS 2 ACKNOWLEDGEMENTS 3 THE CAUVERY RIVER 3 CLIMATE AND VEGETATION 5 TRIBUTARIES 5 COLLECTING STATIONS WITH ECOLOGICAL NOTES 7 MARGINAL AND AQUATIC BIOTA 18 SYSTEMATIC LIST OF CAUVERY FISHES 20 SYSTEMATIC ACCOUNT ••• 28 DISCUSSION 107 CONCLUSIONS AND RECOMMENDATIONS 110 REFERENCES • • . , •• 112 INTRODUCTION Cauvery, Krishna and Godavary rivers constitute the major three ,1.er systems in South India. Geologically they are much older than die Oanga, Indus and Brahmaputra rivers of Northen India. The eco nomic prosperity of the southern states of Andhra Pradesh, Tamil Nadu Kerala and Karnataka is closely intertwined with the water-supply and potentialities of these three rivers. Since historical times their. waters have been extensively utilised for agriculture, fisheries, irrigation and tllYigation purposes. -

RTM-February -2020 Magazine
INSIGHTSIAS IA SIMPLIFYING IAS EXAM PREPARATION RTM COMPILATIONS PRELIMS 2020 FEBRUARY 2020 www.insightsactivelearn.com | www.insightsonindia.com Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 2 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 3 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Table of Contents RTM- REVISION THROUGH MCQS – 1st Feb-2020 ............................................................... 5 RTM- REVISION THROUGH MCQS – 3st Feb-2020 ............................................................. 10 RTM- REVISION THROUGH MCQS – 5th Feb-2020 ............................................................. 16 RTM- REVISION THROUGH MCQS – 6th Feb-2020 ............................................................. 22 RTM- REVISION THROUGH MCQS – 7th Feb-2020 ............................................................. 28 RTM- REVISION THROUGH MCQS – 8th Feb-2020 ............................................................. 34 RTM- REVISION THROUGH MCQS – 10th Feb-2020 ........................................................... 40 RTM- REVISION THROUGH MCQS – 11th Feb-2020 ........................................................... 45 RTM- REVISION THROUGH MCQS – 12th Feb-2020 ........................................................... 52 RTM- REVISION THROUGH MCQS – 13th Feb-2020 .......................................................... -

Urban and Landscape Design Strategies for Flood Resilience In
QATAR UNIVERSITY COLLEGE OF ENGINEERING URBAN AND LANDSCAPE DESIGN STRATEGIES FOR FLOOD RESILIENCE IN CHENNAI CITY BY ALIFA MUNEERUDEEN A Thesis Submitted to the Faculty of the College of Engineering in Partial Fulfillment of the Requirements for the Degree of Masters of Science in Urban Planning and Design June 2017 © 2017 Alifa Muneerudeen. All Rights Reserved. COMMITTEE PAGE The members of the Committee approve the Thesis of Alifa Muneerudeen defended on 24/05/2017. Dr. Anna Grichting Solder Thesis Supervisor Qatar University Kwi-Gon Kim Examining Committee Member Seoul National University Dr. M. Salim Ferwati Examining Committee Member Qatar University Mohamed Arselene Ayari Examining Committee Member Qatar University Approved: Khalifa Al-Khalifa, Dean, College of Engineering ii ABSTRACT Muneerudeen, Alifa, Masters: June, 2017, Masters of Science in Urban Planning & Design Title: Urban and Landscape Design Strategies for Flood Resilience in Chennai City Supervisor of Thesis: Dr. Anna Grichting Solder. Chennai, the capital city of Tamil Nadu is located in the South East of India and lies at a mere 6.7m above mean sea level. Chennai is in a vulnerable location due to storm surges as well as tropical cyclones that bring about heavy rains and yearly floods. The 2004 Tsunami greatly affected the coast, and rapid urbanization, accompanied by the reduction in the natural drain capacity of the ground caused by encroachments on marshes, wetlands and other ecologically sensitive and permeable areas has contributed to repeat flood events in the city. Channelized rivers and canals contaminated through the presence of informal settlements and garbage has exasperated the situation. Natural and man-made water infrastructures that include, monsoon water harvesting and storage systems such as the Temple tanks and reservoirs have been polluted, and have fallen into disuse. -

Tamil Nadu 2014
ENVIRONMENT AND FORESTS DEPARTMENT POLICY NOTE DEMAND No. 54 FORESTS 2014-2015 M.S.M. ANANDAN MINISTER FOR FORESTS © GOVERNMENT OF TAMIL NADU 2014 DEMAND No.54 POLICY NOTE 2014-2015 FOREST DEPARTMENT INTRODUCTION "kâÚU« k©Q« kiyÍ« mâãH‰ fhL« cilaJ mu©" - ÂU¡FwŸ (742) "A fort is that which owns fount of waters crystal clear, an open space, a hill, and shade of beauteous forest near." - Thirukkural (742) The ancient Tamil poets have emphasized the importance of dense forests, clean water and fertile soil in providing ecological security to the mankind. Forests are a complex eco-system which play a dual role of a renewable resource and also as a vital support base for safeguarding the overall environment and ecological balance. It is forest cover that has preserved the soil and its heavy humus that has acted as a porous reservoir to 1 retain water and is gradually releasing it in a sustained flow over a period of time. Trees draw water from the earth crust and release it to the atmosphere by process of transpiration as a part of water cycle. Trees also purify the air by releasing oxygen into the atmosphere after consuming carbon-di-oxide during photosynthesis. The survival and well-being of any nation depends on sustainable social and economic progress, which satisfies the needs of the present generation without compromising the interest of future generation. Spiraling population and increasing industrialization have posed a serious challenge to the preservation of our terrestrial and aquatic ecosystems. Overexploitation of our resources due to rapid population growth has lead to degradation of forests and denudation of agricultural lands. -

Statistical Handbook Tamil Nadu 1983
STATISTICAL HANDBOOK OF TAMIL NADU 1983 NIEPA DC D02122 DEPARTMENT OF STATISTICS MADRAS Price ■ 20.55 t . ' ' . i Sub. Kational TTimT',. NationnI c - ' ' ionl4 * . •: . ,m : 5- .::.--AOOW DOC *Nc..2 ^ I..4 A ............. pafcss. PREFACE The Statistical Handbook of Tatnil Nadu for 1983, presents in compact form, essential factual inforixiation pcrtainingto different sectors of the economy of Tamil Nadu. TTie Handbook is designed to ^e^ve as a ready referencer for those seeking such statistical infOTmation. This Year’s Handbook incorporates for the first time, t he following new Tables: 1. Department of Statistics at a Glance. 2. Consumer price Index Numbers for Rural Tamil Nadu. 3. District Excursion Cenrrts 4. Hon’ble Chief Minister’s Nuiritious Meals Programme. The data presented in this publication have btun collected from various departments of the Central and the State Govcmtnents, Public Sector undertakings and Private institutions- The continuing co-opoation so spontaneously extended by the Heads of Apartments of Government and of Private institutions in furnishing the details is acknowledged with thanks. Suggestions For Airther improvement in future editions of the Handbook are welcome. Place : Madws, V. RAMAMURTHY, Date: 16(h December 1983. Conmissionfr of Statistics. CONTENTS GENERAL INFORMATION PAGSS» Oovemor and Ministers 1 Judges of the High Court 7 Secretaries to Government 9 Commissioners .. 12 District Collectors 13 Tamil Nadu Public Service Comiriss 14 State Planning Commission .. 15 Department of Statistics at a Glance. 17 T am il N adu at a G lance .. 21 SELECT ECONOMIC INDICATORS 1 Population Growth .. .. .. ?5 2 Index Numbers of Agricultural, Ecoaomy .. .......................... 37 3 Index Numbers of Industrial P ro d u c tio n ..................................... -

Cauvery and Mettur Dam Project – an Analysis
Vol 6 Issue 1 July 2016 ISSN No :2231-5063 InternationaORIGINALl M ARTICLEultidisciplinary Research Journal Golden Research Thoughts Chief Editor Dr.Tukaram Narayan Shinde Associate Editor Publisher Dr.Rajani Dalvi Mrs.Laxmi Ashok Yakkaldevi Honorary Mr.Ashok Yakkaldevi Welcome to GRT RNI MAHMUL/2011/38595 ISSN No.2231-5063 Golden Research Thoughts Journal is a multidisciplinary research journal, published monthly in English, Hindi & Marathi Language. All research papers submitted to the journal will be double - blind peer reviewed referred by members of the editorial board.Readers will include investigator in universities, research institutes government and industry with research interest in the general subjects. Regional Editor Dr. T. Manichander International Advisory Board Kamani Perera Mohammad Hailat Hasan Baktir Regional Center For Strategic Studies, Sri Dept. of Mathematical Sciences, English Language and Literature Lanka University of South Carolina Aiken Department, Kayseri Janaki Sinnasamy Abdullah Sabbagh Ghayoor Abbas Chotana Librarian, University of Malaya Engineering Studies, Sydney Dept of Chemistry, Lahore University of Management Sciences[PK] Romona Mihaila Ecaterina Patrascu Spiru Haret University, Romania Spiru Haret University, Bucharest Anna Maria Constantinovici AL. I. Cuza University, Romania Delia Serbescu Loredana Bosca Spiru Haret University, Bucharest, Spiru Haret University, Romania Ilie Pintea, Romania Spiru Haret University, Romania Fabricio Moraes de Almeida Anurag Misra Federal University of Rondonia, Brazil Xiaohua Yang DBS College, Kanpur PhD, USA George - Calin SERITAN Titus PopPhD, Partium Christian Faculty of Philosophy and Socio-Political ......More University, Oradea,Romania Sciences Al. I. Cuza University, Iasi Editorial Board Pratap Vyamktrao Naikwade Iresh Swami Rajendra Shendge ASP College Devrukh,Ratnagiri,MS India Ex - VC. Solapur University, Solapur Director, B.C.U.D. -

Cyclone Warning Division, New Delhi
GOVERNMENT OF INDIA MINISTRY OF EARTH SCIENCES EARTH SYSTEM SCIENCE ORGANIZATION INDIA METEOROLOGICAL DEPARTMENT A Preliminary Report on Depression over Bay of Bengal (13 - 17 November , 2013) CYCLONE WARNING DIVISION, NEW DELHI DECEMBER, 2013 Depression over the Bay of Bengal (13 – 17 November 2013) 1. Introduction A depression formed over southwest and adjoining southeast Bay of Bengal on dated 13th November 2013 near latitude 11.50N and longitude 86.50E, about 700 km east-southeast of Chennai, 770 km east-southeast of Nellore and 730 km east-northeast of Nagapattinam. The system crossed Tamil Nadu coast near Nagapattinam on 0730 UTC of 16th November, 2013. It then moved westwards and weakened gradually a well marked low pressure area over north interior Tamil Nadu at 0530 hrs IST of 17th November, 2013. The salient features of this depression are given below: (i). The depression initially moved westward and then it moved southwestwards and after that it moved west-northwestwards up to north interior Tamil Nadu. (ii) Due to its slow movement over north Tamil Nadu, it caused very good rainfall activity over the region. 2 Monitoring and Prediction: The depression was monitored with satellite, meteorological buoys, coastal, observations and Doppler Weather Radar (DWR) Chennai. The half hourly INSAT/ Kalpana imageries & scattrometer wind and every 10 minutes DWR imageries and products were used for monitoring of depression. Various numerical weather prediction (NWP) models including IMD’s global and meso-scale models were utilized to predict the track and intensity of the depression. The Tropical Cyclone Module in the digitized forecasting system of IMD was utilized for analysis and comparison of various NWP models and decision making process. -
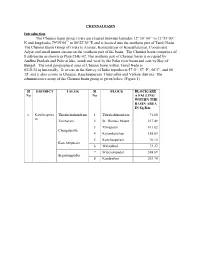
Introduction the Chennai Basin Group Rivers Are Situated Between
CHENNAI BASIN Introduction The Chennai basin group rivers are situated between latitudes 12o 30’ 00’’ to 13 o35’00’’ N and longitudes 79o15’00’’ to 80o22’30’’E and is located into the northern part of Tamil Nadu. The Chennai Basin Group of rivers is Araniar, Korattalaiyar or Kosasthalaiyar, Cooum and Adyar and small minor stream on the southern part of the basin. The Chennai basin comprises of 8 sub basins as shown in Plate:CHE-02. The northern part of Chennai basin is occupied by Andhra Pradesh and Pulicat lake, south and west by the Palar river basin and east by Bay of Bengal. The total geographical area of Chennai basin within Tamil Nadu is 6118.34 sq km totally. It covers in the Survey of India toposheets 57‘O’, 57 ‘P’, 66’C’, and 66 ‘D’ and it also covers in Chennai, Kancheepuram, Thiruvallur and Vellore districts. The administrative setup of the Chennai basin group is given below (Figure 1) Sl DISTRICT TALUK Sl BLOCK BLOCK/ARE No No A FALLING WITHIN THE BASIN AREA IN Sq Km 1. Kancheepura Thirukalunkundram 1 Thirukalukundram 71.60 m Tambaram 2 St. Thomas Mount 217.49 3 Thiruporur 411.82 Chengalpattu 4 Kattankulathur 158.83 5 Kancheepuram 10.14 Kancheepuram 6 Walajabad 73.27 7 Sriperumpudur 248.69 Sriperumpudur 8 Kundrathur 203.70 BLOCK/ARE A FALLING Sl Sl DISTRICT TALUK BLOCK WITHIN THE No No BASIN AREA IN Sq Km Gummidipoond Gummidipoondi 9 420.51 i 10 Minjur 478.69 Ponneri 11 Cholavaram 193.85 12 Puzhal 127.10 Ambattur 13 Villivakkam 210.61 Poonamallee 14 Poonamallee 178.33 2. -

Ii Pullambadi Canal
DEPARTMENT OF ECONOMICS St. JOSEPH’S COLLEGE (Autonomous) (Affiliated to Bharathidasan University, Tiruchirappalli) TIRUCHIRAPPALLI – 620 002. Dr. G. GNANASEKARAN M.A., M.B.A., M.Phil., Ph.D., Head & Research Advisor. CERTIFICATE This is to certify that the thesis entitled “AN ECONOMIC ANALYSIS OF WATER USE EFFICIENCY OF FARMERS IN PULLAMBADI CANAL OF TIRUCHIRAPPALLI AND ARIYALUR DISTRICTS, TAMIL NADU” submitted by Mr. G. IRUTHAYARAJ (Reg. No. 011148 / Ph.D.2 / Economics / F.T. / July 2007) is a bonafide record of research work done by him under my guidance as a full time scholar in the Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli and that the thesis has not previously formed the basis for the award to the candidate of any degree or any other similar title. The thesis is the outcome of personal research work done by the candidate under my overall supervision. (G. GNANASEKARAN) Station: Tiruchirappalli Date : DECLARATION I hereby declare that the work embodied in this thesis has been originally carried out by me under the guidance and supervision of Dr. G. GNANASEKARAN , Head and Research Advisor, Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli - 620 002. This work has not been submitted either in full or in part for any other degree or diploma at any university. (G. IRUTHAYARAJ) Research Scholar Place: Tiruchirappalli Date : ACKNOWLEDGEMENT I wish to place on record the valuable help rendered by various people to complete this dissertation work. I would like to express my profound sense of gratitude to my research adviser and Best Teacher Awardees Dr. G. Gnanasekaran M.A., M.B.A., M.Phil., Ph.D., Head and Associate Professor of Economics, for his stimulating guidance by spending his valuable time with me in sharpening my thinking and analysis, valuable suggestions and continuous encouragement throughout the study. -

Soiled Agriculture and Spoiled Environment: Socio-Economic Impact of Groundwater Pollution in Tamilnadu
Working Paper No. 175 Soiled Agriculture and spoiled environment: Socio-economic impact of groundwater pollution in Tamilnadu by S. Janakarajan Madras Institute of Development Studies 79, Second Main Road, Gandhi Nagar Adyar, Chennai 600 020 September 2002 SOILED AGRICULTUREAND SPOILED ENVIRONMENT: • •SOCIO-ECONOMIC IMPACT OF· GROUNDWATER• POLLUTION IN TAMILNADU• S.JANAKARAJAN Madras Institute of Development Studies Abstract The problem of water pollution• poses a great threat to basic human living. The ramification of pollution is indeed more• severe in the less developed countries that are afflictedwith chronic problems of political instability, lack of political will, high• level of illiteracy, unceasing poverty, increasing degree• of urbanization, lack of basic needs• and basic• infrastructureand women subordination• . The subject matter of groundwater and surfacewater pollution gains further significance due to• economic • .liberaliz.ation policy, to which most underdeveloped countries have been subjected. In other words, the phrase, "sustainable development" emphasized in many international fora, has seemingly no meaning in many underdeveloped countries. • Most river basins, in particular India, are heavily• polluted. In addition, the river basins are stressed due to competing demand for water across different uses and users; there is an intense competition in tapping the good quality water among the sectors such as agriculture, industry and domestic. In the process, milUons of gallons• of good quality water• is transported~ ' from rural to urban areas everyday; the• net result• is the flourishing water trade• and the depletion of• groundwater potential. It causes more anxiety not only because a huge quantity of • water is transported from rural to urban areas,• but also because of the release of comparable quantity of water • as effluent. -

1 Cuddalore District Disaster Management Plan 2017
CUDDALORE DISTRICT DISASTER MANAGEMENT PLAN 2017 1 INTRODUCTION The Cuddalore District Disaster Management Plan for year 2017 is a key for managing disaster related activities and a guidance for emergency management. The information available in DDMP is valuable in terms of its use during disaster. Based on the instructions pertaining to the Sendai Framework Project for Disaster Risk Reduction and on the guidelines of National Institute of Disaster Management (NIDM) formulated by the Central Government and on analysis of history of various disasters that had occurred in this district, this plan has been designed as an action plan rather than a resource book. Utmost attention has been paid to make this Plan Book handy, precise and accurate. During the time of disaster, there may be a delay before outside help arrives. Hence, self-help and assistance from local group is essential in carrying out immediate relief operations. Also, reach to the needy targeted people depends on a prepared community which is alert and informed. Efforts have been made to collect and develop this plan to make it more applicable and effective to handle any type of disaster. Details of inventory resources are given importance in the plan so that during disaster their optimum use can be derived. The important rescue shelters, most necessary equipments, skilled manpower and critical supplies are included in the inventory resources block-wise. Role and responsibility of all departments have been included and the details of control room of various departments, ambulances, blood banks, public health centers, government and private hospitals have been included in this plan.