Dopamine Receptors on Prefrontal Neurons Department of Physiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Equestrian Canada Drug Classification Scheme
Equestrian Canada Drug Classification Scheme This Classification Scheme does not constitute a Prohibited List of banned substances not allowed in horses. Rather, it is designed to allow a hearing panel, after an equine medication violation has been determined, to determine the most appropriate sanction. The drugs, medications and types of substances listed under each Class are not exhaustive. The list in each Class provides examples of drugs, medications and types of substances with similar effects on a horse or that are considered equivalently serious when detected in a horse participating in an EC sanctioned competition. Sanctions will be imposed on a Class by Class basis. Class 1: - Substances which have no place in performing horses. Many of these compounds do not have legitimate medical uses in horses, and the remaining compounds would only be used for severely debilitated animals. Abuse of these compounds is of serious ethical (and many cases, legal) concern. - Most of these compounds are included in Schedules 1 and 3 of the Canadian Controlled Drugs and Substances Act (CDSA) - Examples of Class 1 drugs: o Opioids such as fentanyl, morpine, heroin and codeine o Cocaine o Amphetamine and its derivatives listed in Schedule 3 of the CDSA o Methylphenidate (Ritalin) o Methaqualone Class 2: - These compounds are not appropriate for use around the time of performance. Inappropriate use may alter the horse’s behaviour and ability to perform; medical use of these compounds indicates health status incompatible with athletic competition. -

(12) United States Patent (10) Patent No.: US 6,197,764 B1 Bradley Et Al
USOO6197764B1 (12) United States Patent (10) Patent No.: US 6,197,764 B1 Bradley et al. (45) Date of Patent: *Mar. 6, 2001 (54) CLOZAPINE COMPOSITIONS AND USES FOREIGN PATENT DOCUMENTS THEREOF 0599 576A1 1/1994 (EP). (75) Inventors: Matthews O. Bradley, Laytonsville, 693498 1/1996 (EP). MD (US); Victor E. Shashoua, 61204136 11/1984 (JP). Belmont, MA (US); Charles S. 06-072868 3/1994 (JP). Swindell, Merion; Nigel L. Webb, 6072868 3/1994 (JP). Bryn Mawr, both of PA (US) 7082146 3/1996 (JP). 8151334 6/1996 (JP). (73) Assignee: Protarga, Inc., Conshohocken, PA (US) 9030963 2/1997 (JP). (*) Notice: Subject to any disclaimer, the term of this WO 89/07938 9/1989 (WO). patent is extended or adjusted under 35 WO 96/04001 2/1996 (WO). U.S.C. 154(b) by 0 days. WO 96/22303 7/1996 (WO). WO 96/27380 9/1996 (WO). This patent is Subject to a terminal dis WO98/17325 4/1998 (WO). claimer. OTHER PUBLICATIONS (21) Appl. No.: 08/978,541 (22) Filed: Nov. 26, 1997 Bourat, et al., "Long Chain Esters of Pipotiazine as Lon g-Acting Psychotropic Pro-Drug, Med. Chem. Proc. Int. (51) Int. Cl. .............................................. A61K 31/00 Symp. 5th (1976) pp. 105-114. (52) U.S. Cl. ........................... 514/218; 514/219; 514/220 Lohr, et al., “Neuroleptic-Induced Movement Disorders . (58) Field of Search ..................................... 514/218, 219, ..", Psychiatry, vol. 3, (1989). 514/220 Makino, et al., Chemical Abstracts, vol. 106, No. 12, (56) References Cited (90.177x) issued Mar. 23, 1987, “Pharmaceuticals Permeable to Blood-Brain Barrier'. -

TEXAS RACING COMMISSION January 28, 2021 To
TEXAS RACING COMMISSION P. O. Box 12080, Austin, Texas 78711-2080 8505 Cross Park Drive, Suite 110, Austin, Texas 78754-4552 Phone (512) 833-6699 Fax (512) 833-6907 www.txrc.texas.gov January 28, 2021 To: Stewards, Commission Veterinarians, Test Barn Supervisors, Practicing Veterinarians, Owners, and Trainers From: Chuck Trout, Executive Director Re: Effective February 25, 2021 changes to the following documents: • Permissible Levels of Therapeutic Medications and Naturally Occurring Substances • Equine Medication Classification Policy and Penalty Guidelines • Equine Medication Classification List. This memo is to provide notice that the above listed documents are to be replaced effective this date. The changes include, but are not limited to: • Changes to the list of Permissible Levels of Therapeutic Medications and Naturally Occurring Substances; • Changes to the Equine Medication Classification Policy and Penalty Guidelines; • Changes to the Equine Medication Classification List. These documents are subject to further revision at any time. Test Barn Supervisors - please post this memo and the revised documents in the test barn as soon as possible. Also, please distribute copies of the Permissible Levels of Therapeutic Medications and Naturally Occurring Substances and Equine Medication Classification List to the practicing veterinarians at your racetrack. Licensing Staff - please post this memo and the revised documents where they may be viewed by the public as soon as possible. Copies of these documents will be made available on the Commission's website at http://www.txrc.texas.gov. Attachments: Permissible Levels of Therapeutic Medications and Naturally Occurring Substances Equine Medication Classification Policy and Penalty Guidelines Equine Medication Classification List TEXAS RACING COMMISSION P. -

ARCI Uniform Classification of Foreign Substances
DRUG TESTING STANDARDS AND PRACTICES PROGRAM. Uniform Classification Guidelines for Foreign Substances And Recommended Penalties Model Rule. September 2020 (V.14.2) © ASSOCIATION OF RACING COMMISSIONERS INTERNATIONAL – 2020. Association of Racing Commissioners International 2365 Harrodsburg Road, Suite B-450, Lexington, Kentucky USA 40504 www.arci.com Preamble to the Uniform Classification Guidelines of Foreign Substances The Preamble to the Uniform Classification Guidelines was approved by the RCI Drug Testing and Quality Assurance Program Committee (now the Drug Testing Standards and Practices Program Committee) on August 26, 1991. Minor revisions to the Preamble were made by the Drug Classification subcommittee (now the Veterinary Pharmacologists Subcommittee) on September 3, 1991. "The Uniform Classification Guidelines printed on the following pages are intended to assist stewards, hearing officers and racing commissioners in evaluating the seriousness of alleged violations of medication and prohibited substance rules in racing jurisdictions. Practicing equine veterinarians, state veterinarians, and equine pharmacologists are available and should be consulted to explain the pharmacological effects of the drugs listed in each class prior to any decisions with respect to penalties to be imposed. The ranking of drugs is based on their pharmacology, their ability to influence the outcome of a race, whether or not they have legitimate therapeutic uses in the racing horse, or other evidence that they may be used improperly. These classes of drugs are intended only as guidelines and should be employed only to assist persons adjudicating facts and opinions in understanding the seriousness of the alleged offenses. The facts of each case are always different and there may be mitigating circumstances which should always be considered. -

2021 Equine Prohibited Substances List
2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. LISTED AS SUBSTANCE ACTIVITY BANNED 1-androsterone Anabolic BANNED 3β-Hydroxy-5α-androstan-17-one Anabolic BANNED 4-chlorometatandienone Anabolic BANNED 5α-Androst-2-ene-17one Anabolic BANNED 5α-Androstane-3α, 17α-diol Anabolic BANNED 5α-Androstane-3α, 17β-diol Anabolic BANNED 5α-Androstane-3β, 17α-diol Anabolic BANNED 5α-Androstane-3β, 17β-diol Anabolic BANNED 5β-Androstane-3α, 17β-diol Anabolic BANNED 7α-Hydroxy-DHEA Anabolic BANNED 7β-Hydroxy-DHEA Anabolic BANNED 7-Keto-DHEA Anabolic CONTROLLED 17-Alpha-Hydroxy Progesterone Hormone FEMALES BANNED 17-Alpha-Hydroxy Progesterone Anabolic MALES BANNED 19-Norandrosterone Anabolic BANNED 19-Noretiocholanolone Anabolic BANNED 20-Hydroxyecdysone Anabolic BANNED Δ1-Testosterone Anabolic BANNED Acebutolol Beta blocker BANNED Acefylline Bronchodilator BANNED Acemetacin Non-steroidal anti-inflammatory drug BANNED Acenocoumarol Anticoagulant CONTROLLED Acepromazine Sedative BANNED Acetanilid Analgesic/antipyretic CONTROLLED Acetazolamide Carbonic Anhydrase Inhibitor BANNED Acetohexamide Pancreatic stimulant CONTROLLED Acetominophen (Paracetamol) Analgesic BANNED Acetophenazine Antipsychotic BANNED Acetophenetidin (Phenacetin) Analgesic BANNED Acetylmorphine Narcotic BANNED Adinazolam Anxiolytic BANNED Adiphenine Antispasmodic BANNED Adrafinil Stimulant 1 December 2020, Lausanne, Switzerland 2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). -

Drug/Substance Trade Name(S)
A B C D E F G H I J K 1 Drug/Substance Trade Name(s) Drug Class Existing Penalty Class Special Notation T1:Doping/Endangerment Level T2: Mismanagement Level Comments Methylenedioxypyrovalerone is a stimulant of the cathinone class which acts as a 3,4-methylenedioxypyprovaleroneMDPV, “bath salts” norepinephrine-dopamine reuptake inhibitor. It was first developed in the 1960s by a team at 1 A Yes A A 2 Boehringer Ingelheim. No 3 Alfentanil Alfenta Narcotic used to control pain and keep patients asleep during surgery. 1 A Yes A No A Aminoxafen, Aminorex is a weight loss stimulant drug. It was withdrawn from the market after it was found Aminorex Aminoxaphen, Apiquel, to cause pulmonary hypertension. 1 A Yes A A 4 McN-742, Menocil No Amphetamine is a potent central nervous system stimulant that is used in the treatment of Amphetamine Speed, Upper 1 A Yes A A 5 attention deficit hyperactivity disorder, narcolepsy, and obesity. No Anileridine is a synthetic analgesic drug and is a member of the piperidine class of analgesic Anileridine Leritine 1 A Yes A A 6 agents developed by Merck & Co. in the 1950s. No Dopamine promoter used to treat loss of muscle movement control caused by Parkinson's Apomorphine Apokyn, Ixense 1 A Yes A A 7 disease. No Recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. It is often claimed that BZP was originally Benzylpiperazine BZP 1 A Yes A A synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals. -
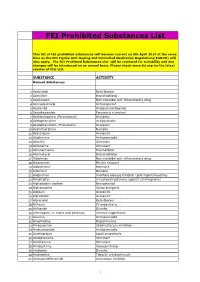
FEI Prohibited Substances List
FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply. The FEI Prohibted Substances List will be reviewed for suitability and any changes will be introduced on an annual basis. Please check www.fei.org for the latest version of this List. SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin Non-steroidal anti-inflammatory drug 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/antipyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic 8 Acetophenazine Antipsychotic 9 Acetophenetidin (Phenacetin) Analgesic 10 Acetylmorphine Narcotic 11 Adinazolam Anxiolytic 12 Adiphenine Antispasmodic 13 Adrafinil Stimulant 14 Adrenaline Stimulant 15 Adrenochrome Haemostatic 16 Aformoterol Bronchodilator 17 Alclofenac Non-steroidal anti-inflammatory drug 18 Alcuronium Muscle relaxant 19 Aldosterone Hormone 20 Alfentanil Narcotic 21 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 22 Almotriptan 5-hydroxatryptamine agonist (antimigraine) 23 Alphadolone acetate Neurosteriod 24 Alphaprodine Opiod analgesic 25 Alpidem Anxiolytic 26 Alprazolam Anxiolytic 27 Alprenolol Beta blocker 28 Althesin IV anaesthetic 29 Althiazide Diuretic 30 Altrenogest (in males and geldings) Oestrus suppression 31 Alverine Antispasmodic 32 Amantadine Dopaminergic 33 Ambenonium Cholinesterase inhibition 34 Ambucetamide Antispasmodic 35 Amethocaine Local anaesthetic 36 Amfepramone Stimulant 37 Amfetaminil Stimulant 38 Amidephrine Vasoconstrictor 39 Amiloride Diuretic 40 Amineptine Tricyclic antidepressant 41 Aminoglutehthamide Aromatase inhibitor 1 FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply. -

(Amendment) 810 KAR 8:020. Drug, Medication, and Substance Classifica
PUBLIC PROTECTION CABINET KENTUCKY HORSE RACING COMMISSION (Amendment) 810 KAR 8:020. Drug, medication, and substance classification schedule [and withdrawal guidelines]. RELATES TO: KRS 230.215, 230.225, 230.240, 230.260, 230.265, 230.290, 230.320, 230.370 STATUTORY AUTHORITY: KRS 230.215(2), 230.225, 230.240(2), 230.260, 230.320, 230.370 NECESSITY, FUNCTION, AND CONFORMITY: KRS 230.215(2) authorizes the Kentucky Horse Racing Commission (the “commission”) to promulgate administrative regulations prescribing conditions under which all legitimate horse racing and wagering thereon is conducted in Kentucky. KRS 230.240(2) requires the commission to promulgate administrative regulations restricting or prohibiting the administration of drugs or stimulants or other improper acts to horses prior to the horse participating in a race. This administrative regulation establishes the drug classification schedule in effect in Kentucky[and the withdrawal guidelines] for permitted drugs, medications, and substances that may be administered to race horses competing in Kentucky. Section 1. The Kentucky Horse Racing Commission Uniform Drug, Medication, and Sub- stance Classification Schedule.[, KHRC 8-020-1,] (1) This administrative regulation shall establish the respective classifications of all substances contained herein[therein. The Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian, KHRC 8-020-2, shall provide certain mandatory treatment requirements and guidance and advice on withdrawal intervals as contained herein]. (2)(a) Class A drugs, medications, and substances are those that: (1) Have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration; or (2) Lack approval by the United States Food and Drug Administration, but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. -

Uniform Classification Guidelines for Foreign Substances, Version 7.00
Association of Racing Commissioners International, Inc. Drug Testing Standards and Practices Program Model Rules Guidelines Uniform Classification Guidelines for Foreign Substances and Recommended Penalties and Model Rule Version 7.00 Revised January 2014 Association of Racing Commissioners International, Inc. Uniform Classification Guidelines for Foreign Substances Table of Contents Preamble to the Uniform Classification Guidelines of Foreign Substances ..................................................................................... ii Notes Regarding Classification Guidelines ...................................................................................................................................... ii Classification Criteria ....................................................................................................................................................................... iii Classification Definitions ................................................................................................................................................................. iv Drug Classification Scheme ............................................................................................................................................................. vi Alphabetical Substance List ............................................................................................................................................................... 1 Listing by Classification .................................................................................................................................................................. -
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Molecules 2016, 21, 75; doi:10.3390/molecules21010075 S1 of S110 Supplementary Materials: Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs Fei Mao 1, Wei Ni 1, Xiang Xu 1, Hui Wang 1, Jing Wang 1, Min Ji 1 and Jian Li * Table S1. Common names, indications, CAS Registry Numbers and molecular formulas of 6891 approved drugs. Common Name Indication CAS Number Oral Molecular Formula Abacavir Antiviral 136470-78-5 Y C14H18N6O Abafungin Antifungal 129639-79-8 C21H22N4OS Abamectin Component B1a Anthelminithic 65195-55-3 C48H72O14 Abamectin Component B1b Anthelminithic 65195-56-4 C47H70O14 Abanoquil Adrenergic 90402-40-7 C22H25N3O4 Abaperidone Antipsychotic 183849-43-6 C25H25FN2O5 Abecarnil Anxiolytic 111841-85-1 Y C24H24N2O4 Abiraterone Antineoplastic 154229-19-3 Y C24H31NO Abitesartan Antihypertensive 137882-98-5 C26H31N5O3 Ablukast Bronchodilator 96566-25-5 C28H34O8 Abunidazole Antifungal 91017-58-2 C15H19N3O4 Acadesine Cardiotonic 2627-69-2 Y C9H14N4O5 Acamprosate Alcohol Deterrant 77337-76-9 Y C5H11NO4S Acaprazine Nootropic 55485-20-6 Y C15H21Cl2N3O Acarbose Antidiabetic 56180-94-0 Y C25H43NO18 Acebrochol Steroid 514-50-1 C29H48Br2O2 Acebutolol Antihypertensive 37517-30-9 Y C18H28N2O4 Acecainide Antiarrhythmic 32795-44-1 Y C15H23N3O2 Acecarbromal Sedative 77-66-7 Y C9H15BrN2O3 Aceclidine Cholinergic 827-61-2 C9H15NO2 Aceclofenac Antiinflammatory 89796-99-6 Y C16H13Cl2NO4 Acedapsone Antibiotic 77-46-3 C16H16N2O4S Acediasulfone Sodium Antibiotic 80-03-5 C14H14N2O4S Acedoben Nootropic 556-08-1 C9H9NO3 Acefluranol Steroid