Solvent Similarity Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Dehydration of Cellulose to Levoglucosenone Using Polar Aprotic Solvents Cite This: Energy Environ
Energy & Environmental Science View Article Online PAPER View Journal | View Issue Dehydration of cellulose to levoglucosenone using polar aprotic solvents Cite this: Energy Environ. Sci., 2015, 8,1808 Fei Cao,ab Thomas J. Schwartz,a Daniel J. McClelland,a Siddarth H. Krishna,a James A. Dumesica and George W. Huber*a Herein, we report an approach to produce levoglucosenone (LGO) from cellulose in yields up to 51% under Received 2nd February 2015, mild reaction conditions (170–230 1C; 5–20 mM H2SO4) using polar, aprotic solvents such as tetrahydro- Accepted 18th May 2015 furan(THF).LGOcanbeusedtomakeawidevarietyof chemicals from biomass. The water content and DOI: 10.1039/c5ee00353a solvent used in the reaction system control the product distribution. LGO is produced from the dehydration of levoglucosan (LGA). LGA is produced from cellulose depolymerization. Increasing the water content leads www.rsc.org/ees to the production of 5-hydroxymethyl furfural (HMF), obtaining a maximum HMF yield of 30%. Creative Commons Attribution 3.0 Unported Licence. Broader context This article describes the sustainable conversion of cellulose to levoglucosenone (LGO) using mild reaction conditions in the liquid phase. LGO is an attractive, biomass-derived platform molecule that can be used for the renewable production of pharmaceuticals, commodity chemicals such as 1,6-hexanediol (HDO), and green solvents such as dihydrolevoglucosenone. The co-production of such high-value species has the potential to significantly improve the overall economic feasibility of a biorefinery. Notably, dihydrolevoglucosenone, also known as Cyrenes, is a polar, aprotic solvent with properties similar to N- methylpyrrolidone and sulfolane, both of which are traditionally obtained from fossil-based resources. -

Aldrichimica Acta
VOLUME 51, NO. 1 | 2018 ALDRICHIMICA ACTA The Spectacular Resurgence of Electrochemical Redox Reactions in Organic Synthesis Carbon–Carbon π Bonds as Conjunctive Reagents in Cross-Coupling The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. MakInG sTrIDes In Genome EDITInG: THe DIGITaL, CHemIcaL, anD BIoLogIcaL Dear Fellow Researchers: You may not know that MilliporeSigma can make DNA from scratch, using 100% synthetic chemical methods—no living cells and no fermentation necessary. Our DNA synthesis facilities in The Woodlands (Texas) and Haverhill (U.K.) receive thousands of DNA orders daily from scientists all over the world. A small piece of DNA, one hundred bases long, gives scientists 4100 possibilities using only the fundamental letters of our genetic code (A,C,G,T). Included within these options is the opportunity for a researcher to open a common text editor on his or her PC and design a 100-base piece of DNA. This piece of DNA could help study diseases ranging from sickle cell anemia and blindness to cystic fibrosis by using CRISPR. To get started, scientists cut this 100-base DNA text from the text editor and paste it into MilliporeSigma’s online ordering system. The corresponding DNA piece that likely has never existed before is then delivered to them in 24–48 hours. Once received, this piece of DNA is mixed with CRISPR and living cells, and, with a “blast” of electricity, these synthetic chemical pieces activate the cell to edit the genome. Learn more about our CRISPR gene editing portfolio at SigmaAldrich.com/CRISPR Sincerely yours, Udit Batra, Ph.D. -

Ethers, Ether-Alcohols, Ether-Phenols, Ether
29-IV Sub-Chapter IV ETHERS, ALCOHOL PEROXIDES, ETHER PEROXIDES, KETONE PEROXIDES, EPOXIDES WITH A THREE-MEMBERED RING, ACETALS AND HEMIACETALS, AND THEIR HALOGENATED, SULPHONATED, NITRATED OR NITROSATED DERIVATIVES 29.09 - Ethers, ether-alcohols, ether-phenols, ether-alcohol-phenols, alcohol peroxides, ether peroxides, ketone peroxides (whether or not chemically defined), and their halogenated, sulphonated, nitrated or nitrosated derivatives. - Acyclic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives : 2909.11 - - Diethyl ether 2909.19 - - Other 2909.20 - Cyclanic, cyclenic or cycloterpenic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives 2909.30 - Aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives - Ether-alcohols and their halogenated, sulphonated, nitrated or nitrosated derivatives : 2909.41 - - 2,2’-Oxydiethanol (diethylene glycol, digol) 2909.43 - - Monobutyl ethers of ethylene glycol or of diethylene glycol 2909.44 - - Other monoalkylethers of ethylene glycol or of diethylene glycol 2909.49 - - Other 2909.50 - Ether-phenols, ether-alcohol-phenols and their halogenated, sulphonated, nitrated or nitrosated derivatives 2909.60 - Alcohol peroxides, ether peroxides, ketone peroxides and their halogenated, sulphonated, nitrated or nitrosated derivatives (A) ETHERS Ethers may be considered as alcohols or phenols in which the hydrogen atom of the hydroxyl group is replaced by a hydrocarbon radical (alkyl or aryl). They have the general formula : (R-O-R1), where R and R1 may be the same or different. These ethers are very stable, neutral substances. If the radicals belong to the acyclic series, the ether is also acyclic; cyclic radicals give cyclic ethers. The first ether in the acyclic series is gaseous, but others are volatile liquids with a characteristic odour of ether; the higher members are liquids or sometimes solids. -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Bio-Solvents: Synthesis, Industrial Production and Applications Novisi K
Chapter Bio-Solvents: Synthesis, Industrial Production and Applications Novisi K. Oklu, Leah C. Matsinha and Banothile C.E. Makhubela Abstract Solvents are at the heart of many research and industrial chemical processes and consumer product formulations, yet an overwhelming number are derived from fossils. This is despite societal and legislative push that more products be produced from carbon-neutral resources, so as to reduce our carbon footprint and environmental impact. Biomass is a promising renewable alternative resource for producing bio-solvents, and this review focuses on their extraction and synthesis on a laboratory and large scale. Starch, lignocellulose, plant oils, animal fats and proteins have been combined with creative synthetic pathways, novel technolo- gies and processes to afford known or new bio-derived solvents including acids, alkanes, aromatics, ionic liquids (ILs), furans, esters, ethers, liquid polymers and deep eutectic solvents (DESs)—all with unique physiochemical properties that warrant their use as solvation agents in manufacturing, pharmaceutical, cosmet- ics, chemicals, energy, food and beverage industries, etc. Selected bio-solvents, conversion technologies and processes operating at commercial and demonstration scale including (1) Solvay’s Augeo™ SL 191 renewable solvent, (2) Circa Group’s Furacell™ technology and process for making levoglucosenone (LGO) to produce dihydrolevoglucosenone (marketed as Cyrene™), (3) Sappi’s Xylex® technology and demonstration scale processes that aim to manufacture precursors for bio- solvents and (4) Anellotech’s Bio-TCat™ technology and process for producing benzene, toluene and xylenes (BTX) are highlighted. Keywords: bio-solvents, renewable resources, green chemistry, biorefinery, biomass 1. Introduction Air quality deterioration, environmental, health and safety issues have raised serious concerns over continued processing of fossil-based feedstocks in producing chemical products such as fuels and solvents. -

Dihydrolevoglucosenone (Cyrene™), a Bio-Based Solvent for Liquid-Liquid Extraction Applications Thomas Brouwer, and Boelo Schuur ACS Sustainable Chem
Subscriber access provided by UNIV TWENTE Article Dihydrolevoglucosenone (Cyrene™), a Bio-based Solvent for Liquid-Liquid Extraction Applications Thomas Brouwer, and Boelo Schuur ACS Sustainable Chem. Eng., Just Accepted Manuscript • DOI: 10.1021/ acssuschemeng.0c04159 • Publication Date (Web): 31 Aug 2020 Downloaded from pubs.acs.org on September 16, 2020 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts. is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036 Published by American Chemical Society. -
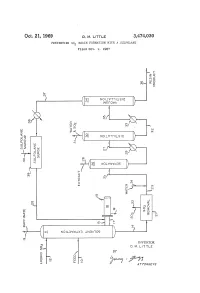
G ( NOW T LSO )
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane. -

Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals.--2Nd Ed
Second Edition Physical-Chemical Properties and Environmental Fate for Organic Chemicals Volume I Introduction and Hydrocarbons Volume II Halogenated Hydrocarbons Volume III HANDBOOK OF HANDBOOK Oxygen Containing Compounds Volume IV Nitrogen and Sulfur Containing Compounds and Pesticides © 2006 by Taylor & Francis Group, LLC Second Edition Physical-Chemical Properties and Environmental Fate for Organic Chemicals Volume I Introduction and Hydrocarbons Volume II Halogenated Hydrocarbons Volume III Oxygen Containing Compounds HANDBOOK OF HANDBOOK Volume IV Nitrogen and Sulfur Containing Compounds and Pesticides Donald Mackay Wan Ying Shiu Kuo-Ching Ma Sum Chi Lee Boca Raton London New York A CRC title, part of the Taylor & Francis imprint, a member of the Taylor & Francis Group, the academic division of T&F Informa plc. © 2006 by Taylor & Francis Group, LLC Published in 2006 by CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2006 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10987654321 International Standard Book Number-10: 1-56670-687-4 (Hardcover) International Standard Book Number-13: 978-1-56670-687-2 (Hardcover) Library of Congress Card Number 2005051402 This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. -

Japanese Flavoring Agents As Food Additives
Japanese Flavoring Agents as Food Additives In Japan, synthetic flavoring agents are allowed to be used only when they are designated by the Minister of Health, Labour and Welfare as food additives under the Japanese Food Sanitation Act. Currently, we have identified 170 chemical substances which are commonly used as flavorings as shown in Table 1. Table 2 lists 18 groups which are also from the official list of “designated additives” and contain 3004 additional flavor materials. Each of the 18 groups in Table 2 contains substances that are similar in chemical structure. For links to a complete listing of the Japanese additives used in food: (a) Designated additives, (b) Existing food additives, (c) Natural flavoring agents and (d) Ordinary foods used as food additives - go to http://www.mhlw.go.jp/english/topics/foodsafety/foodadditives/index.html Check for Flavoring updates at http://www.jffma-jp.org/english/information.html Provided with Updated Revisions (as of April 2015) By Leffingwell & Associates Table 1. Designated additives used as flavoring substances Compound Synonym or Old name CAS Acesulfame Potassium 55589-62-3 Acetaldehyde (New as of 2006.05.16) ethanal 75-07-0 Acetophenone acetophenone 98-86-2 Acetic acid, Glacial 64-19-7 Adipic Acid 124-04-9 714229-20-6 Advantame (New as of 2015.02.20) 245650-17-3 DL-Alanine 302-72-7 Allyl cyclohexylpropionate allyl cyclohexanepropionate 2705-87-5 Allyl hexanoate allyl hexanoate 123-68-2 Allyl isothiocyanate allyl isothiocyanate 57503 (3-Amino-3-carboxypropyl)dimethylsulfonium chloride -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -
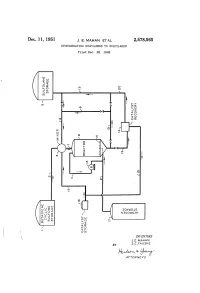
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound.