G ( NOW T LSO )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -
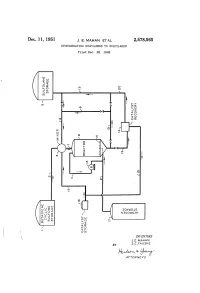
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -

A Field Pilot Study on Treating Groundwater Contaminated with Sulfolane Using UV/H2O2
water Article A Field Pilot Study on Treating Groundwater Contaminated with Sulfolane Using UV/H2O2 Linlong Yu 1, Sobhan Iranmanesh 1, Ian Keir 2 and Gopal Achari 1,* 1 Department of Civil Engineering, University of Calgary, 2500 University Drive NW, Calgary, AB T2N 1N4, Canada; [email protected] (L.Y.); [email protected] (S.I.) 2 Bonavista Energy Corporation (formerly), 525 8 Ave SW Suite 1500, Calgary, AB T2P 1G1, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-403-220-6599 Received: 3 April 2020; Accepted: 21 April 2020; Published: 23 April 2020 Abstract: Sulfolane is an emerging contaminant in the groundwater and soil nearby gas plants, which has attracted much attention from many researchers and regulatory agencies in the past ten years. In this paper, a field pilot-scale ultraviolet (UV)/hydrogen peroxide (H2O2) system was investigated for treating sulfolane contaminated groundwater. Different groundwater, as well as different operational parameters such as influent sulfolane concentration, H2O2 dosage, and water flow rates, were studied. The results showed that a pilot-scale UV/H2O2 system can successfully treat sulfolane contaminated groundwater in the field, although the presence of iron and other groundwater limited the process efficiency. The lowest electrical energy per order of reduction for 3 1 treating sulfolane in groundwater by using the pilot-scale UV/H2O2 system was 1.4 kWh m− order− . The investigated sulfolane initial concentrations and the water flow rates did not impact the sulfolane degradation. The enhancement of sulfolane degradation in an open reservoir by adding ozone was not observed in this study. -

Licensed Technologies Innovative | Proven | Value-Adding
Licensed Technologies Innovative | Proven | Value-adding lyondellbasell.com Saudi Ethylene and Polyethylene Company (SEPC), Al-Jubail Industrial City, Kingdom of Saudi Arabia LyondellBasell is the world’s third-largest independent chemical company. We have annual revenues of approximately $30.8 billion and more than 14,000 employees worldwide. Our vertically integrated facilities, broad product portfolio, manufacturing flexibility, superior technology base and reputation for operational excellence enable us to deliver exceptional value to our customers across the petrochemical chain – from refining to advanced product applications. Product diversity and vertical integration allow LyondellBasell to capture value at every step of the petrochemical chain. Natural Gas Wellhead Crude Liquids Capturing value along the chain Refining Refining Fuels Olefins Olefins Crackers Olefins Aromatics T echnology Propylene Olefin OxyFuels Polyethylene Polypropylene Polybutene-1 Acetyls Ethylene Oxide Styrene Derivatives Oxide Glycols, Glycol Ethers Glycols 2nd Level PP Catalloy Derivatives Compounding Butanediol Glycol Ethers Refining & OxyFuels Olefins & Polyolefins Olefins & Polyolefins Intermediates & Derivatives Technology Americas Europe, Asia & International 2 A global leader in polyolefins and chemicals technology, production and marketing About LyondellBasell LyondellBasell’s technologies are some of the Global capacity positions most reliable, efficient and cost effective in the With major administrative offices in Houston, world. With over 280 licensed -

Sulfolane Mixtures As Ambient Aprotic Polar Solvents
(19) TZZ __T (11) EP 2 778 158 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 17.09.2014 Bulletin 2014/38 C07D 333/48 (2006.01) (21) Application number: 14159793.0 (22) Date of filing: 14.03.2014 (84) Designated Contracting States: (71) Applicant: Advanced Technology Materials, Inc. AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Danbury, CT 06810 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (72) Inventor: Cooper, Emanuel, I. Designated Extension States: Scarsdale, NY 10583 (US) BA ME (74) Representative: ABG Patentes, S.L. (30) Priority: 14.03.2013 US 201361781820 P Avenida de Burgos, 16D Edificio Euromor 28036 Madrid (ES) (54) Sulfolane mixtures as ambient aprotic polar solvents (57) An improved solvent containing sulfolane and at least one dialkyl sulfone, preferably dimethyl sulfone, wherein the improved solvent is a liquid at room temperature and can be used for reaction media and electrochemistry. EP 2 778 158 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 778 158 A1 Description FIELD 5 [0001] The present invention relates generally to an improved solvent for reaction media and electrochemistry. DESCRIPTION OF THE RELATED ART [0002] Sulfolane is a well-known industrial solvent used most often for extractive distillation and to purify natural gas, 10 and also as an aprotic reaction medium and for electrochemical applications. Advantageously, sulfolane has a high dielectric constant, a low toxicity and a very low skin penetration rate, is stable, water soluble, chemically compatible with strong acids and oxidants and bases, has a low decomposition rate, and a very high flash point. -

NTP Research Concept: Sulfolane
NTP Research Concept: Sulfolane Project Leader Chad Blystone, PhD, DABT NIEHS/DNTP/Toxicology Branch Background and Rationale Sulfolane (CAS# 126-33-0) was nominated by the State of Alaska’s Department of Environmental Conservation and Department of Health and Social Services, Mayor of the Fairbanks North Star Borough, Alaska, a Senator from the Alaska State Legislature, and the Agency for Toxic Substances and Disease Registry. Sulfolane is a polar solvent primarily used in the refining of natural gas and petroleum. Other uses include fractionalization of wood tars and curing of epoxy resins. Production of sulfolane was between 10 and 50 million pounds in the United States according the US EPA’s 2006 Inventory Update Rule reporting. Recently sulfolane has been detected in well water within the town of North Pole, Alaska. A plume was identified in groundwater, originating from a petroleum refinery, and the highest concentration measured in wells outside the refinery was 269 ppb as of January 2011. Due to the contamination, residents were being provided bottled water for use and additional wells were created outside the plume area. Sulfolane contamination has also been identified within Canada in areas near sites of natural gas or petroleum refining and may be present as a groundwater contaminant near other sites of refining around the county. A few studies have demonstrated the uptake of sulfolane into vegetation at contaminated sites. In an occupational setting, inhalation and dermal contact could be the primary route(s) of exposure. Animal toxicity data for sulfolane are limited. An inhalation study of aerosolized sulfolane investigated potential toxic effects in various species (rats, guinea pigs, dogs, squirrel monkeys), but experimental detail and design are limited (1). -

Optimization of the Sulfolane Extraction Plant Based on Modeling and Simulation
Korean J. Chem. Eng., 17(6), 712-718 (2000) Optimization of the Sulfolane Extraction Plant Based on Modeling and Simulation Yu-Jung Choi, Tae-In Kwon and Yeong-Koo Yeo† Department of Chemical Engineering, Hanyang University, 17 Haengdang-dong, Sungdong-ku, Seoul 133-791, Korea (Received 14 August 2000 • accepted 24 October 2000) Abstract−An optimization system, based on modeling and simulation, was developed for a sulfolane extraction plant. The primary objective of the operation of this plant is to increase benzene composition, which is mostly affected by the recycle streams in the plant. In this work the optimal recycle streams were identified and resulting product com- positions were evaluated. In the optimization, suitable parametric models for each process unit were obtained first from the steady-state rigorous modeling and simulation of the sulfolane extraction plant. The parametric models were then employed to develop the optimization system based on the SQP scheme. Results of simulations show promise for fur- ther economic improvements over present operation states. Key words: Sulfolane, Extraction Plant, Simulation, SQP, Optimization INTRODUCTION The bottom products of the wash column and the extract-di- stillation column are fed into the solvent recovery column which is A BTX (Benzene-Toluene-Xylene) plant consists of four unit operated in the range of 40-200 oC. Water and aromatics are ob- plants including a sulfolane extraction plant. In this plant, aromat- tained as the overhead product, which in turn is separated by den- ics including BTX and non-aromatics are extracted from hydroge- sity difference, and the resulting water is recycled to the wash col- nated crude oils by using sulfolane as the solvent. -

Supporting Information Green Chemistry Final
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Supporting Information Guidelines to Design Electrolytes for Lithium-ion Batteries: Environmental impact, Physicochemical and Electrochemical Properties Benjamin Flamme,a Gonzalo Rodriguez Garcia,b† Marcel Weil,c† Mansour Haddad,a Phannarath Phansavath,a Virginie Ratovelomanana-Vidal,a Alexandre Chagnesd a) PSL Research University, Chimie ParisTech - CNRS, Institut de Recherche de Chimie Paris, 11 rue Pierre et Marie Curie, 75005, Paris, France b) Helmholtz Institute Ulm (HIU) Electrochemical Energy Storage, Helmholtzstraße 11, 89081 Ulm, Germany. Current address: Helmholtz Zentrum Dresden Rossendorf (HZDR) Bautzner Landstraße 400, 01328 Dresden, Germany c) Institute for Technology Assessment and System Analysis (ITAS), Karlstraße 11, 76133 Karlsruhe, Germany. d) University of Lorraine, GeoRessources Lab, UMR CNRS 7359, 2 Rue Doyen Marcel Roubault,TSA 70605, 54518 Vandoeuvre Les Nancy, France. † Karlsruhe Institute of Technology (KIT), P.O. Box 3640, 76021 Karlsruhe, Germany [email protected] for discussion about the physicochemistry and electrochemistry in lithium-ion batteries [email protected] for discussion about Life Cycle Assessment 1 Acronyms Acronyms Usual name IUPAC name 1,3-DL Dioxolane 1,3-dioxolane 2FEA Fluoroethyl acetate 2-fluoroethyl acetate 2FEP Fluoroethyl propionate 2-fluoroethyl propionate 2FPMC 2-fluoropropyl methyl carbonate 2-fluoropropyl methyl carbonate 2Me-1,3-DL 2-methyl dioxolane -

Acute Toxicity and Skin Irritant Properties of Sulfolane V
Brit. J. industr. Med., I966, 23, 302 Acute Toxicity and Skin Irritant Properties of Sulfolane V. K. H. BROWN, L. W. FERRIGAN, and D. E. STEVENSON From 'Shell' Research Ltd., Tunstall Laboratory, Sittingbourne, Kent Sulfolane is a useful industrial solvent. The oral LD50 values were: rats, I-7 to 2s7 g./kg.; mice, I-9 to 2. 5 g./kg., all deaths taking place within 24 hours. Rats showed no effects after 3-8 g./kg. had been applied to the skin. The compound did not irritate or sensitize the skins of guinea-pigs or rabbits and, undiluted, was almost without action on the eyes of rabbits. Because of its solvent properties, chemical conditions does not react with acids or bases. Substitu- inertness, and cheapness, sulfolane is a useful selec- tion in the 3,4-position is relatively easy, and sulfolane tive solvent in liquid-liquid and liquid-vapour can be reduced to 3,4-sulfolene (Reid, I960). Sulfolane extraction processes used for the separation of com- is almost odourless but at 220°C. it decomposes slowly with the formation of sulphur dioxide. Sulfolane is pounds with different degrees of saturation and completely miscible with a wide range of different polarity, e.g., for the extraction of aromatic com- liquids, e.g., water, acetone, glycerol, toluene, and many pounds from petroleum. It is also a good solvent oils. Some of its other more important physical pro- for quaternary ammonium salts, nitrocellulose, poly- perties are given in Table I. vinylchloride, polystyrene, and for protein or amide-type fibres. TABLE I Very little information is available on the toxicity and possible irritancy of sulfolane, and, because of SOME PHYSICAL PROPERTIES OF TECHNICAL SULFOLANE Physical Property the importance of this material in industry, it was Molecular weight I20-i6 decided to investigate these properties. -

Executive Summary on Sulfolane Research Report (PDF)
SULFOLANE TECHNICAL ASSISTANCE AND EVALUATION REPORT EXECUTIVE SUMMARY This report summarizes research of sulfolane’s physical and chemical properties, industrial ap- plications, corrosivity, breakdown and attenuation processes, and case histories involving sulfo- lane spills. The information included in this report is intended for readers to better understand how sulfolane is used in the oil and gas industry, its potential to cause corrosion in process equipment and piping, sulfolane regulations, and case histories of sulfolane spills and any clean- up that was completed. Sulfolane is an organic compound that is readily soluble in water; it prefers to dissolve in water rather than stay in its pure form or attach to soil particles. It is also soluble, although to a lesser extent, in hydrocarbons (components of fuel). In its pure form, sulfolane is a clear, colorless liq- uid that is heavier than water. It does not readily evaporate like other solvents do, such as paint thinners or benzene. Nor does it like to adsorb – stick to – soils, the way benzene does. The high solubility of sulfolane in water means that when it is released into the environment, it tends to move toward groundwater. Once in groundwater, it spreads out and becomes diluted as it travels with the groundwater flow. Sulfolane is a man-made industrial solvent used in a wide variety of applications including oil refining, natural gas production, the production of insecticides, herbicides, and fungicides, li- thium batteries, pharmaceuticals, printer ink, circuit board cleaning solutions, semiconductors, and soap. At Flint Hills, sulfolane is used as the primary solvent in a sulfolane extraction unit that removes the aromatics such as benzene, toluene, and xylenes from refined oil so that they can later be added at very specific concentrations for each type of gasoline produced at the refinery.