Role of Solvents in CO2 Capture Processes : the Review of Selection and Design Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Solubility and Aggregation of Selected Proteins Interpreted on the Basis of Hydrophobicity Distribution
International Journal of Molecular Sciences Article Solubility and Aggregation of Selected Proteins Interpreted on the Basis of Hydrophobicity Distribution Magdalena Ptak-Kaczor 1,2, Mateusz Banach 1 , Katarzyna Stapor 3 , Piotr Fabian 3 , Leszek Konieczny 4 and Irena Roterman 1,2,* 1 Department of Bioinformatics and Telemedicine, Jagiellonian University—Medical College, Medyczna 7, 30-688 Kraków, Poland; [email protected] (M.P.-K.); [email protected] (M.B.) 2 Faculty of Physics, Astronomy and Applied Computer Science, Jagiellonian University, Łojasiewicza 11, 30-348 Kraków, Poland 3 Institute of Computer Science, Silesian University of Technology, Akademicka 16, 44-100 Gliwice, Poland; [email protected] (K.S.); [email protected] (P.F.) 4 Chair of Medical Biochemistry—Jagiellonian University—Medical College, Kopernika 7, 31-034 Kraków, Poland; [email protected] * Correspondence: [email protected] Abstract: Protein solubility is based on the compatibility of the specific protein surface with the polar aquatic environment. The exposure of polar residues to the protein surface promotes the protein’s solubility in the polar environment. The aquatic environment also influences the folding process by favoring the centralization of hydrophobic residues with the simultaneous exposure to polar residues. The degree of compatibility of the residue distribution, with the model of the concentration of hydrophobic residues in the center of the molecule, with the simultaneous exposure of polar residues is determined by the sequence of amino acids in the chain. The fuzzy oil drop model enables the quantification of the degree of compatibility of the hydrophobicity distribution Citation: Ptak-Kaczor, M.; Banach, M.; Stapor, K.; Fabian, P.; Konieczny, observed in the protein to a form fully consistent with the Gaussian 3D function, which expresses L.; Roterman, I. -

Solubility & Density
Natural Resources - Water WHAT’S SO SPECIAL ABOUT WATER: SOLUBILITY & DENSITY Activity Plan – Science Series ACTpa025 BACKGROUND Project Skills: Water is able to “dissolve” other substances. The molecules of water attract and • Discovery of chemical associate with the molecules of the substance that dissolves in it. Youth will have fun and physical properties discovering and using their critical thinking to determine why water can dissolve some of water. things and not others. Life Skills: • Critical thinking – Key vocabulary words: develops wider • Solvent is a substance that dissolves other substances, thus forming a solution. comprehension, has Water dissolves more substances than any other and is known as the “universal capacity to consider solvent.” more information • Density is a term to describe thickness of consistency or impenetrability. The scientific formula to determine density is mass divided by volume. Science Skills: • Hypothesis is an explanation for a set of facts that can be tested. (American • Making hypotheses Heritage Dictionary) • The atom is the smallest unit of matter that can take part in a chemical reaction. Academic Standards: It is the building block of matter. The activity complements • A molecule consists of two or more atoms chemically bonded together. this academic standard: • Science C.4.2. Use the WHAT TO DO science content being Activity: Density learned to ask questions, 1. Take a clear jar, add ¼ cup colored water, ¼ cup vegetable oil, and ¼ cup dark plan investigations, corn syrup slowly. Dark corn syrup is preferable to light so that it can easily be make observations, make distinguished from the oil. predictions and offer 2. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Carbon Dioxide Capture by Chemical Absorption: a Solvent Comparison Study
Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study by Anusha Kothandaraman B. Chem. Eng. Institute of Chemical Technology, University of Mumbai, 2005 M.S. Chemical Engineering Practice Massachusetts Institute of Technology, 2006 SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING PRACTICE AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2010 © 2010 Massachusetts Institute of Technology All rights reserved. Signature of Author……………………………………………………………………………………… Department of Chemical Engineering May 20, 2010 Certified by……………………………………………………….………………………………… Gregory J. McRae Hoyt C. Hottel Professor of Chemical Engineering Thesis Supervisor Accepted by……………………………………………………………………………….................... William M. Deen Carbon P. Dubbs Professor of Chemical Engineering Chairman, Committee for Graduate Students 1 2 Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study by Anusha Kothandaraman Submitted to the Department of Chemical Engineering on May 20, 2010 in partial fulfillment of the requirements of the Degree of Doctor of Philosophy in Chemical Engineering Practice Abstract In the light of increasing fears about climate change, greenhouse gas mitigation technologies have assumed growing importance. In the United States, energy related CO2 emissions accounted for 98% of the total emissions in 2007 with electricity generation accounting for 40% of the total1. Carbon capture and sequestration (CCS) is one of the options that can enable the utilization of fossil fuels with lower CO2 emissions. Of the different technologies for CO2 capture, capture of CO2 by chemical absorption is the technology that is closest to commercialization. While a number of different solvents for use in chemical absorption of CO2 have been proposed, a systematic comparison of performance of different solvents has not been performed and claims on the performance of different solvents vary widely. -

THE SOLUBILITY of GASES in LIQUIDS INTRODUCTION the Solubility Data Project Aims to Make a Comprehensive Search of the Lit- Erat
THE SOLUBILITY OF GASES IN LIQUIDS R. Battino, H. L. Clever and C. L. Young INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the lit erature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uni form format. The data for each system are evaluated and where data of suf ficient accuracy are available values recommended and in some cases a smoothing equation suggested to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are pUblished on consecutive pages. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solUbility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solUbility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the critical temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solu bility depending on the particular range of pressure considered and the par ticular worker concerned. The difficulty partly stems from our inability to rigorously distinguish between a gas, a vapor, and a liquid, which has been discussed in numerous textbooks. -

Biotransformation: Basic Concepts (1)
Chapter 5 Absorption, Distribution, Metabolism, and Elimination of Toxics Biotransformation: Basic Concepts (1) • Renal excretion of chemicals Biotransformation: Basic Concepts (2) • Biological basis for xenobiotic metabolism: – To convert lipid-soluble, non-polar, non-excretable forms of chemicals to water-soluble, polar forms that are excretable in bile and urine. – The transformation process may take place as a result of the interaction of the toxic substance with enzymes found primarily in the cell endoplasmic reticulum, cytoplasm, and mitochondria. – The liver is the primary organ where biotransformation occurs. Biotransformation: Basic Concepts (3) Biotransformation: Basic Concepts (4) • Interaction with these enzymes may change the toxicant to either a less or a more toxic form. • Generally, biotransformation occurs in two phases. – Phase I involves catabolic reactions that break down the toxicant into various components. • Catabolic reactions include oxidation, reduction, and hydrolysis. – Oxidation occurs when a molecule combines with oxygen, loses hydrogen, or loses one or more electrons. – Reduction occurs when a molecule combines with hydrogen, loses oxygen, or gains one or more electrons. – Hydrolysis is the process in which a chemical compound is split into smaller molecules by reacting with water. • In most cases these reactions make the chemical less toxic, more water soluble, and easier to excrete. Biotransformation: Basic Concepts (5) – Phase II reactions involves the binding of molecules to either the original toxic molecule or the toxic molecule metabolite derived from the Phase I reactions. The final product is usually water soluble and, therefore, easier to excrete from the body. • Phase II reactions include glucuronidation, sulfation, acetylation, methylation, conjugation with glutathione, and conjugation with amino acids (such as glycine, taurine, and glutamic acid). -
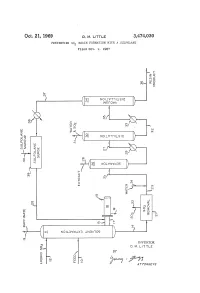
G ( NOW T LSO )
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane. -

Design Guidelines for Carbon Dioxide Scrubbers I
"NCSCTECH MAN 4110-1-83 I (REVISION A) S00 TECHNICAL MANUAL tow DESIGN GUIDELINES FOR CARBON DIOXIDE SCRUBBERS I MAY 1983 REVISED JULY 1985 Prepared by M. L. NUCKOLS, A. PURER, G. A. DEASON I OF * Approved for public release; , J 1"73 distribution unlimited NAVAL COASTAL SYSTEMS CENTER PANAMA CITY, FLORIDA 32407 85. .U 15 (O SECURITY CLASSIFICATION OF TNIS PAGE (When Data Entered) R O DOCULMENTATIONkB PAGE READ INSTRUCTIONS REPORT DOCUMENTATION~ PAGE BEFORE COMPLETING FORM 1. REPORT NUMBER 2a. GOVT AQCMCSION N (.SAECIP F.NTTSChALOG NUMBER "NCSC TECHMAN 4110-1-83 (Rev A) A, -NI ' 4. TITLE (and Subtitle) S. TYPE OF REPORT & PERIOD COVERED "Design Guidelines for Carbon Dioxide Scrubbers '" 6. PERFORMING ORG. REPORT N UMBER A' 7. AUTHOR(&) 8. CONTRACT OR GRANT NUMBER(S) M. L. Nuckols, A. Purer, and G. A. Deason 9. PERFORMING ORGANIZATION NAME AND ADDRESS 10. PROGRAM ELEMENT, PROJECT. TASK AREA 6t WORK UNIT NUMBERS Naval Coastal PanaaLSystems 3407Project CenterCty, S0394, Task Area Panama City, FL 32407210,WrUnt2 22102, Work Unit 02 II. CONTROLLING OFFICE NAME AND ADDRE1S t2. REPORT 3ATE May 1983 Rev. July 1985 13, NUMBER OF PAGES 69 14- MONI TORING AGENCY NAME & ADDRESS(if different from Controtling Office) 15. SECURITY CLASS. (of this report) UNCLASSIFIED ISa. OECL ASSI FICATION/DOWNGRADING _ __N•AEOULE 16. DISTRIBUTION STATEMENT (of thia Repott) Approved for public release; distribution unlimited. 17. DISTRIBUTION STATEMENT (of the abstract entered In Block 20, If different from Report) IS. SUPPLEMENTARY NOTES II. KEY WORDS (Continue on reverse side If noceassry and Identify by block number) Carbon Dioxide; Scrubbers; Absorption; Design; Life Support; Pressure; "Swimmer Diver; Environmental Effects; Diving., 20. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -

Raoult's Law – Partition Law
BAE 820 Physical Principles of Environmental Systems Henry’s Law - Raoult's Law – Partition law Dr. Zifei Liu Biological and Agricultural Engineering Henry's law • At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. Pi = KHCi • Where Pi is the partial pressure of the gaseous solute above the solution, C is the i William Henry concentration of the dissolved gas and KH (1774-1836) is Henry’s constant with the dimensions of pressure divided by concentration. KH is different for each solute-solvent pair. Biological and Agricultural Engineering 2 Henry's law For a gas mixture, Henry's law helps to predict the amount of each gas which will go into solution. When a gas is in contact with the surface of a liquid, the amount of the gas which will go into solution is proportional to the partial pressure of that gas. An equivalent way of stating the law is that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. the solubility of gases generally decreases with increasing temperature. A simple rationale for Henry's law is that if the partial pressure of a gas is twice as high, then on the average twice as many molecules will hit the liquid surface in a given time interval, Biological and Agricultural Engineering 3 Air-water equilibrium Dissolution Pg or Cg Air (atm, Pa, mol/L, ppm, …) At equilibrium, Pg KH = Caq Water Caq (mol/L, mole ratio, ppm, …) Volatilization Biological and Agricultural Engineering 4 Various units of the Henry’s constant (gases in water at 25ºC) Form of K =P/C K =C /P K =P/x K =C /C equation H, pc aq H, cp aq H, px H, cc aq gas Units L∙atm/mol mol/(L∙atm) atm dimensionless -3 4 -2 O2 769 1.3×10 4.26×10 3.18×10 -4 4 -2 N2 1639 6.1×10 9.08×10 1.49×10 -2 3 CO2 29 3.4×10 1.63×10 0.832 Since all KH may be referred to as Henry's law constants, we must be quite careful to check the units, and note which version of the equation is being used.