Supporting Information Green Chemistry Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Of Grignard Reagent Formation. the Surface Nature of the Reaction
286 Ace. Chem. Res. 1990,23, 286-293 Mechanism of Grignard Reagent Formation. The Surface Nature of the Reaction H. M. WALBORSKY Dittmer Laboratory of Chemistry, Florida State University, Tallahassee, Florida 32306 Received February 23, 1990 (Revised Manuscript Received May 7, 1990) The reaction of organic halides (Br, C1, I) with mag- Scheme I nesium metal to yield what is referred to today as a Kharasch-Reinmuth Mechanism for Grignard Reagent Grignard reagent has been known since the turn of the Formation century,' The name derives from its discoverer, Nobel (1)(Mg0)AMg*)2y + RX 4 [(M~'~(MQ')~~-,('MQX)+ R.] + laureate Victor Grignard. How this reagent is formed, (Mgo)x-2(MQ')2~MgX)(MgR) that is, how a magnesium atom is inserted into a car- bon-halogen bond, is the subject of this Account. ('4 (Ms0),-*(M9')2~MgX)(MgR) + + (Mg0)x-dMg*)2y+2 + 2RMgX RX + Mg - RMgX Kharasch and Reinmuth,, persuaded by the work of late under the same conditions gave Itl = 6.2 X s-l. Another system that meets the above criterion is the Gomberg and Bachmad as well as by product analyses of many Grignard formation reactions that existed in vinyl system. The lack of reactivity of vinyl halides toward SN1reactions is well-known and is exemplified the literature prior to 1954,speculated that the reaction involved radicals and that the radical reactions might by the low solvolysis rate of 2-propenyl triflate5 in 80% involve "surface adherent radicals, at least in part". The ethanol at 25 OC, kl being 9.8 X s-l. -

Methoxyethane, Physical Properties, Safety, MSDS, Enthalpy, Gas Liquid
(c) Bürkle GmbH 2021 Important Important information The tables “Chemical resistance of plastics”, “Plastics and their properties” and “Viscosity of liquids" as well as the information about chemical resistance given in the particular product descriptions have been drawn up based on information provided by various raw material manufacturers. These values are based solely on laboratory tests with raw materials. Plastic components produced from these raw materials are frequently subject to influences that cannot be recognized in laboratory tests (temperature, pressure, material stress, effects of chemicals, construction features, etc.). For this reason the values given are only to be regarded as being guidelines. In critical cases it is essential that a test is carried out first. No legal claims can be derived from this information; nor do we accept any liability for it. A knowledge of the chemical and mechanical resistance alone is not sufficient for the evaluation of the usability of a product. For example, the regulations concerning flammable liquids (explosion prevention) must also be taken into consideration. Copyright This table has been published and updated by Bürkle GmbH, D-79415 Bad Bellingen as a work of reference. This Copyright clause must not be removed. The table may be freely passed on and copied, provided that the information about the publisher is retained. Extensions, additions and translations If your own experiences with materials and media could be used to extend this table then we would be pleased to receive any additional information. Please send an E-Mail to [email protected]. We would also like to receive translations into other languages. -
G ( NOW T LSO )
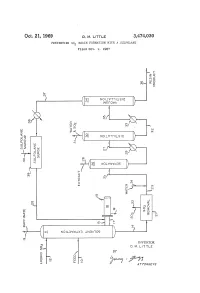
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane. -

United States Patent (19) 11) Patent Number: 4,585,788 Helsley Et Al
United States Patent (19) 11) Patent Number: 4,585,788 Helsley et al. 45) Date of Patent: Apr. 29, 1986 54 6,11-DIHYDRODIBENZB,EOXEPIN ACETIC ACIDS AND DERVATIVES FOREIGN PATENT DOCUMENTS 48-389 6/1972 Japan . 75 Inventors: Grover C. Helsley, Pottersville; 000425 7/1972 Japan. Arthur R. McFadden, East Brunswick, both of N.J.; David Primary Examiner-Norma S. Milestone Hoffman, North Kingstown, R.I. Attorney, Agent, or Firm-Curtis, Morris & Safford 57 ABSTRACT (73) Assignee: American Hoechst Corporation, 6,11-Dihydrodibenzb,eoxepin-acetic acids and deriva Somerville, N.J. tives having the general formula 21 Appl. No.: 459,774 1 22 Fied: Apr. 10, 1974 210 X Yn C-Z Related U.S. Application Data St. 5 k 63 Continuation-in-part of Ser. No. 394,801, Sep. 6, 1973, abandoned. (51) Int. Cl." .................. C07D 313/12; A61K 31/335 are prepared by multi-step sequences. X is C=O, CHCl, 52) U.S. C. ..................................... 514/450; 549/354 CHBr, CH2 or CHOR; Y is alkyl or alkoxy of 1 to 4 (58) Field of Search ........................ 260/333; 549/354; carbon atoms, halogen or trifluoromethyl; n is 0, 1, 2 or 514/450 3; Z is COOR5, CH2OR5, CONR25 or CONHOR5; and Rl-Rs are hydrogen or alkyl of 1 to 4 carbon atoms. 56) References Cited These compounds and the physiologically tolerable U.S. PATENT DOCUMENTS salts thereof are useful as antiinflammatory and analge 3,702,852 11/1972 Yale et al. ........................... 260/333 sic agents. 3,714,201 1/1973 Yale et al. ........................... 260/333 3,758,528 9/1973 Malen et al. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

List of Criteria and Selected Air Contaminants to Be Used for Public Notice, with Spanish Translations. COMMON AIR CONTAMINANTS
List of Criteria and Selected Air Contaminants To Be Used for Public Notice, with Spanish Translations. COMMON AIR CONTAMINANTS: English Spanish NOX NOX (óxidos de nitrógeno) CO monóxido de carbono PM materia en partículas SO2 or SOX SO2 or SOX (óxidos de azufre) Halogens halógenos Hydrocarbons hidrocarburos Inorganic Acids ácidos inorgánicos Inorganic Bases bases inorgánicas Metals metales Carbon Compounds compuestos de carbono Carbon compounds including (but not limited to) : Benzene, Methyl Ethyl Ketone and Freon 11. Compuestos de carbono incluyendo (pero no limitándose a): Benceno, Metiletilcetona y Freón 11. OTHER AIR CONTAMINANTS English - Spanish Air Contaminant Glossary for Spanish Public Notice This list contains numerous air contaminants, mostly as listed in the ESL (= Effects Screening Levels) lists of 6/22/92 and updates of 1994. The Spanish translated names are capitalized for trademarks. In many such cases, there is no translation and the English word must be used. English Name Spanish Name Abamectin Abamectin Abate Abate acenaphthene acenafteno acetal acetal acetaldehyde acetaldehído acetamide acetamida acetic acid acido acético acetic anhydride anhídrido acético acetoacetoxyethyl methacrylate,2- 2-metacrilato acetoacetoxietílico acetone acetona acetone cyanohydrin cianohidrina de acetona acetonitrile acetonitrilo acetophenone acetofenona acetylaminofluorene,2- 2-acetilaminofluoreno acetyl chloride cloruro acetílico [AOAQG01/962 v2] Page 1 of 52 acetylene acetileno acetylene dichloride dicloruro de acetileno (1,2-dichloroethylene) -

Environmental Protection Agency Pt. 98, Subpt. A, Table A–1
Environmental Protection Agency Pt. 98, Subpt. A, Table A–1 to verify the amount of GHG emis- through the electronic GHG reporting sions, and failure to calculate GHG tool, must be submitted through that emissions following the methodologies tool. If not specified, requests, notifica- specified in this part. Each day of a tions or communications shall be sub- violation constitutes a separate viola- mitted to the following address: tion. (a) For U.S. mail. Director, Climate § 98.9 Addresses. Change Division, 1200 Pennsylvania Ave., NW., Mail Code: 6207J, Wash- All requests, notifications, and com- ington, DC 20460. munications to the Administrator pur- (b) For package deliveries. Director, suant to this part must be submitted Climate Change Division, 1310 L St, electronically and in a format as speci- NW., Washington, DC 20005. fied by the Administrator. For exam- ple, any requests, notifications and [74 FR 56374, Oct. 30, 2009, as amended at 76 communications that can be submitted FR 73900, Nov. 29, 2011] TABLE A–1 TO SUBPART A OF PART 98—GLOBAL WARMING POTENTIALS [100-Year Time Horizon] Global warming Name CAS No. Chemical formula potential (100 yr.) Chemical-Specific GWPs Carbon dioxide ................................................................... 124–38–9 CO2 ............................................... 1 a Methane ............................................................................. 74–82–8 CH4 ............................................... 25 a Nitrous oxide ..................................................................... -

United States Patent Office Patented Mar
3,083,236 United States Patent Office Patented Mar. 26, 1963 2 anol; or aliphatic ethers including diethoxyethane and di 3,083,236 methoxyethane, and hydrocarbons such as cyclohexane. HYDROGENATHON OF SEHYDROXY-METHYL The solution containing a small amount of a suitable FURFURAL catalyst, preferably copperchromite catalyst, is heated to Torleif Uine, Warrenville, John D. Garber, Westfield, and Robert E. Jones, Rahway, N.J., assignors to Merck & 5 the appropriate temperature for some time while under a Co., Inc., Rahway, N.J., a corporation of New Jersey hydrogen pressure varying from about 200 to 20,000 p.s.i., No Drawing. Filled Sept. 10, 1958, Ser. No. 760,056 preferably from 280 to 6000 p.s.i. The copperchromite 2 Claims. (C. 250-635) may also contain barium oxide which has a beneficial effect. About 10% by weight of catalyst based on the This invention relates to 5-hydroxymethyl furfural HMF is generally satisfactory. Palladium on charcoal (HMF) and has for its object the provision of an im was found to be an effective co-catalyst when mixed with proved process for forming derivatives from this com copperchromite in producing 1,6-hexanediol. pound. In accordance with our invention we subject The pressures used have varied from about 200 to HMF to catalytic hydrogenation to form a series of de 20,000 p.si. without noticeable additional benefits in the rivative compounds in an efficient operation. high part of the pressure range, especially above 6000 The invention involves the discovery that HMF can be p.s. i. After the reaction, the product is cooled, filtered subjected to hydrogenation with a suitable catalyst and to remove the catalyst, and heated under a vacuum to hydrogen under various conditions to produce different remove the solvent. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years.