Water Quality Guidelines for Sulfolane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -
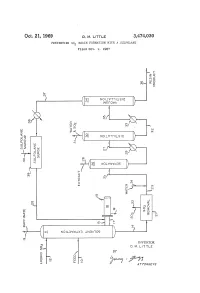
G ( NOW T LSO )
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane. -

Asymmetric Desymmetrization of Oxetanes for the Synthesis of Chiral
Angewandte Research Articles Chemie International Edition:DOI:10.1002/anie.201910917 Asymmetric Catalysis German Edition:DOI:10.1002/ange.201910917 Asymmetric Desymmetrization of Oxetanes for the Synthesis of Chiral Tetrahydrothiophenes and Tetrahydroselenophenes Renwei Zhang+,Wengang Guo+,Meng Duan+,K.N.Houk,* and Jianwei Sun* Abstract: Chiral tetrahydrothiophenes and tetrahydroseleno- phenes are highly useful structural units.Described here is anew catalytic asymmetric approach for their synthesis.With asuitable chiral Brønsted acid catalyst, an oxetane desymmet- rization by awell-positioned internal sulfur or selenium nucleophile proceeded efficiently to generate all-carbon qua- ternary stereocenters with excellent enantioselectivities.Taming the sulfur and selenium nucleophile in the form of athioester and selenoester,respectively,iscrucial to the success of this work. This approach also allows the facile synthesis of chiral tetrahydrothiopyrans.Mechanistic studies,including DFT calculations,suggested an intramolecular acyl-transfer path- way.Utilities of the chiral products are also demonstrated. Introduction Figure 1. Useful compounds containing chiral tetrahydrothiophene and tetrahydroselenopheneunits. Chiral organosulfur and organoselenium compounds play important roles in biological processes,organic synthesis,and asymmetric hydrogenation of substituted thiophenes (Sche- medicinal chemistry.[1] In particular, chiral tetrahydrothio- me 1a).[6] However,unfortunately,this elegant process suffers phenes and tetrahydroselenophenes are well-known subunits from alimited scope:good enantioselectivity was only in awide range of natural products and bioactive molecules observed for asmall number of thiophenes bearing specific that exhibit abroad spectrum of important biological aryl substituent at the 2-position. activities (e.g., I–V,Figure 1).[2] Moreover,such chiral units Moreover,the nature of this approach excludes the embedded in arelatively rigid five-membered ring structure possibility of generating aquaternary stereogenic center. -

Sodium-Mediated Magnesiation of Thiophene and Tetrahydrothiophene: Structural Contrasts with Furan and Tetrahydrofuran
Sodium-mediated Magnesiation of Thiophene and Tetrahydrothiophene: Structural Contrasts with Furan and Tetrahydrofuran Victoria L. Blair, Alan R. Kennedy, Robert E. Mulvey and Charles T. O’Hara* V. L. Blair, Dr. A. R. Kennedy, Prof. R. E. Mulvey, Dr. C. T. O’Hara WestCHEM, Department of Pure and Applied Chemistry University of Strathclyde Glasgow, G1 1XL (U.K.) Fax: (+44) 141 548 4822 E-mail: [email protected]; [email protected] Sulfur-containing heterocycles are currently attracting a great deal of interest in several diverse fields. For instance, substituted tetrahydrothiophenes,[1] have received considerable attention due to their extremely wide-ranging chemical and biological applications.[2] These include their use as potent α-glucosidase inhibitors,[3] as an inhibitor of copper amine [4] [5] oxidases and as selective A3 agonists and antagonists. In addition, they have been utilized in chemical transformations, such as catalytic asymmetric epoxidation, catalytic intramolecular cyclopropanation, and asymmetric metal catalysis hydrogenation.[6] From a nanochemical perspective, the adsorption chemistries and physical properties of various thiophenes and tetrahydrothiophenes on gold surfaces have recently come to the fore.[7] Polythiophenes are also key compounds in modern materials research, currently utilised in for example the fabrication of semi-conducting, fluorescent, and electronic and optoelectronic materials.[8]In this work, metallation (exchange of a hydrogen atom with a metal atom) of the parent heterocycles, tetrahydrothiophene (THT) and thiophene is considered. Metallation is one of the most fundamental reactions in modern day synthesis and is a key tool in the preparation of functionalised aromatic and heterocyclic compounds. -

Acceptor Ligands in Stabilizing and Assembling Pt Or Ir Carbonyl Clusters Simona El Afefey
UNIVERSITA’ DEGLI STUDI DI MILANO Scuola di Dottorato in Scienze Chimiche Dipartimento di Chimica PhD in Industrial Chemistry XXVCycle Electron-donor and -acceptor ligands in stabilizing and assembling Pt or Ir carbonyl clusters (Chim 03) Simona El Afefey Supervisor: Prof. ALESSANDRO CERIOTTI Coordinator: Prof. DOMINIQUE ROBERTO A.A. 2011/2012 Simona El Afefey Electron-donor and –acceptor ligands in stabilizing and assembling Pt or Ir carbonyl clusters ________________________________________________________________________________________________ Abstract ............................................................................................................................................... 7 Introduction ..................................................................................................................................... 21 1.1 Carbon Monoxide as ligand .................................................................................................... 21 1.2 General considerations on metal carbonyl clusters ............................................................. 25 1.2.1 Synthetic aspects of metal carbonyl clusters .............................................................. 26 1.2.2 Synthetic methods .......................................................................................................... 28 1.2.3 Direct reductive-carbonylation ................................................................................... 29 1.2.4 Thermal methods .......................................................................................................... -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -
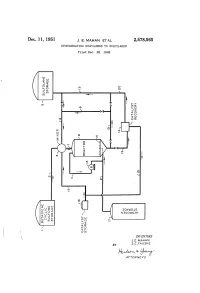
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -

Thiophene Hydrodesulfurization Catalysis on Supported Ru Clusters: Mechanism and Site Requirements for Hydrogenation and Desulfurization Pathways
Journal of Catalysis 273 (2010) 245–256 Contents lists available at ScienceDirect Journal of Catalysis journal homepage: www.elsevier.com/locate/jcat Thiophene hydrodesulfurization catalysis on supported Ru clusters: Mechanism and site requirements for hydrogenation and desulfurization pathways Huamin Wang, Enrique Iglesia * Department of Chemical Engineering, University of California and Chemical Sciences Division, E.O. Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA article info abstract Article history: Kinetic and isotopic methods were used to probe elementary steps and site requirements for thiophene Received 9 April 2010 hydrogenation and desulfurization on Ru metal clusters. Turnover rates for these reactions were unaf- Revised 27 May 2010 fected by whether samples were treated in H2 or H2S to form metal and sulfide clusters, respectively, Accepted 28 May 2010 before reaction. These data, taken together with the rate and extent of sulfur removal from used samples Available online 1 July 2010 during contact with H2, indicate that active structures consist of Ru metal clusters saturated with chem- isorbed sulfur at temperatures, pressures, and H2S levels relevant to hydrodesulfurization catalysis. Turn- Keywords: over rates and isotopic data over a wide range of H ,HS, and thiophene pressures are consistent with Hydrodesulfurization 2 2 elementary steps that include quasi-equilibrated H and H S heterolytic dissociation and thiophene bind- Thiophene 2 2 1 4 Ruthenium ing with g (S) or g coordination onto sulfur vacancies. We conclude that hydrogenation proceeds via d+ d+ 4 Kinetic addition of protons (H , as –S–H from H2 or H2S dissociation) to g thiophene species, while desulfur- 1 dÀ Kinetic isotope effects ization involves C–S activation in g (S) species aided by H species formed via H2 dissociation. -

A Field Pilot Study on Treating Groundwater Contaminated with Sulfolane Using UV/H2O2
water Article A Field Pilot Study on Treating Groundwater Contaminated with Sulfolane Using UV/H2O2 Linlong Yu 1, Sobhan Iranmanesh 1, Ian Keir 2 and Gopal Achari 1,* 1 Department of Civil Engineering, University of Calgary, 2500 University Drive NW, Calgary, AB T2N 1N4, Canada; [email protected] (L.Y.); [email protected] (S.I.) 2 Bonavista Energy Corporation (formerly), 525 8 Ave SW Suite 1500, Calgary, AB T2P 1G1, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-403-220-6599 Received: 3 April 2020; Accepted: 21 April 2020; Published: 23 April 2020 Abstract: Sulfolane is an emerging contaminant in the groundwater and soil nearby gas plants, which has attracted much attention from many researchers and regulatory agencies in the past ten years. In this paper, a field pilot-scale ultraviolet (UV)/hydrogen peroxide (H2O2) system was investigated for treating sulfolane contaminated groundwater. Different groundwater, as well as different operational parameters such as influent sulfolane concentration, H2O2 dosage, and water flow rates, were studied. The results showed that a pilot-scale UV/H2O2 system can successfully treat sulfolane contaminated groundwater in the field, although the presence of iron and other groundwater limited the process efficiency. The lowest electrical energy per order of reduction for 3 1 treating sulfolane in groundwater by using the pilot-scale UV/H2O2 system was 1.4 kWh m− order− . The investigated sulfolane initial concentrations and the water flow rates did not impact the sulfolane degradation. The enhancement of sulfolane degradation in an open reservoir by adding ozone was not observed in this study. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Nonflammable Gas Mixture: Argon / Hydrogen Sulfide / N-Butane / Tetrahydrothiophene / Thiophene Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Argon / Hydrogen Sulfide / N-Butane / Tetrahydrothiophene / Thiophene Other means of : Not available. identification Product type : Gas. Product use : Synthetic/Analytical chemistry. SDS # : 022841 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Do not depend on odor to detect presence of gas. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation.