Makhahlela N 29910749.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecology and Epidemiology of Campylobacter Jejuni in Broiler Chickens
Ecology and Epidemiology of Campylobacter jejuni in Broiler Chickens A DISSERTATION SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA BY Hae Jin Hwang IN PARTIALL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Dr. Randall Singer, Dr. George Maldonado June 2019 © Hae Jin Hwang, 2019 Acknowledgements I would like to sincerely thank my advisor, Dr. Randall Singer, for his intellectual guidance and support, great patience, and mentorship, which made this dissertation possible. I would also like to thank Dr. George Maldonado for his continuous encouragement and support. I would further like to thank my thesis committee, Dr. Richard Isaacson and Dr. Timothy Church, for their guidance throughout my doctoral training. I thank all my friends and colleagues I met over the course of my studies. I am especially indebted to my friends, Dr. Kristy Lee, Dr. Irene Bueno Padilla, Dr. Elise Lamont, Madhumathi Thiruvengadam, Dr. Kaushi Kanankege and Dr. Sylvia Wanzala, for their support and friendship. Heartfelt gratitude goes to my family, for always believing in me, encouraging me and helping me get through the difficult and stressful times during my studies. Lastly, I thank Sven and Bami for being the best writing companions I could ever ask for. i Abstract Campylobacteriosis, predominantly caused by Campylobacter jejuni, is a common, yet serious foodborne illness. With consumption and handling of poultry products as the most important risk factor of campylobacteriosis, reducing Campylobacter contamination in poultry products is considered the best public health intervention to reduce the burden and costs associated with campylobacteriosis. To this end, there is a need to improve our understanding of epidemiology and ecology of Campylobacter jejuni in poultry. -

Babela Massiliensis, a Representative of a Widespread Bacterial
Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, Samia Benamar, Didier Raoult, Eugene V. Koonin, Bernard La Scola To cite this version: Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, et al.. Babela mas- siliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae. Biology Direct, BioMed Central, 2015, 10 (13), 10.1186/s13062-015-0043-z. hal-01217089 HAL Id: hal-01217089 https://hal-amu.archives-ouvertes.fr/hal-01217089 Submitted on 19 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Pagnier et al. Biology Direct (2015) 10:13 DOI 10.1186/s13062-015-0043-z RESEARCH Open Access Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier1, Natalya Yutin2, Olivier Croce1, Kira S Makarova2, Yuri I Wolf2, Samia Benamar1, Didier Raoult1, Eugene V Koonin2 and Bernard La Scola1* Abstract Background: Only a small fraction of bacteria and archaea that are identifiable by metagenomics can be grown on standard media. -
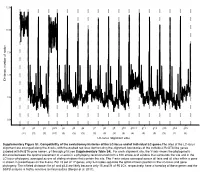
LC-Locus Alignment Sites Distance, Number of Nodes Supplementary
12.5 10.0 7.5 5.0 Distance, number of nodes 2.5 0.0 g1 g2 g3 g3.5 g4 g5 g6 g7 g8 g9 g10 g10.1 g11 g12 g13 g14 g15 (11) (3) (3) (10) (3) (3) (3) (3) (3) (3) (3) (4) (3) (3) (3) (2) (3) LC-locus alignment sites Supplementary Figure S1. Compatibility of the evolutionary histories of the LC-locus and of individual LC genes.The sites of the LC-locus alignment are arranged along the X-axis, with the dashed red lines demarcating the alignment boundaries of the individual RcGTA-like genes (labeled with RcGTA gene names, g1 through g15; see Supplementary Table S4). For each alignment site, the Y-axis shows the phylogenetic distance between the optimal placement of a taxon in a phylogeny reconstructed from a 100 amino-acid window that surrounds the site and in the LC-locus phylogeny, averaged across all sliding windows that contain the site. The Y-axis values averaged across all taxa and all sites within a gene is shown in parentheses on the X-axis. For 15 out of 17 genes, only 2-4 nodes separate the optimal taxon position in the LC-locus and gene phylogeny. The inflated distances for g1 and g3.5 are likely because only 15 and 21 of 95 LCs, respectively, have a homolog of these genes and the SSPB analysis is highly sensitive to missing data (Berger et al. 2011). a. Bacteria Unassigned Thermotogae Tenericutes Synergistetes Spirochaetes Proteobacteria ylum Planctomycetes h p Firmicutes Deferribacteres Cyanobacteria Chloroflexi Bacteroidetes Actinobacteria Acidobacteria 1(11,750) 2(1,750) 3(2,538) 4(168) 5(51) 6(54) 7(43) 8(32) 9(26) 10(40) 11(33) 12(198) 13(173) 14(101) 15(98) 16(43) 17(114) Number of rcc01682−rcc01698 homologs in a cluster b. -

Anaplasma Platys Diagnosis in Dogs
Anaplasma platys Diagnosis in Dogs: Comparison Between Morphological and Molecular Tests Renata Fernandes Ferreira, VMD, MSc1 Aloysio de Mello Figueiredo Cerqueira, VMD, MSc, DSc2 Ananda Müller Pereira, VMD1 Cecília Matheus Guimarães BSc2 Alexandre Garcia de Sá, VMD, MSc1 Fabricio da Silva Abreu, VMD, MSc1 Carlos Luiz Massard, VMD, MSc, PhD3 Nádia Regina Pereira Almosny, VMD, MSc, PhD1 1Departamento de Patologia e Clínica Veterinária Universidade Federal Fluminense Niterói, Rio de Janeiro, Brazil 2Departamento de Microbiologia e Parasitologia Universidade Federal Fluminense Niterói, Rio de Janeiro, Brazil 3Departamento de Parasitologia Animal Universidade Federal Rural do Rio de Janeiro Seropédica, Rio de Janeiro, Brazil KEY WORDS: Anaplasma platys, PCR, ickettsia helminthoeca (PCR1). The second inclusions stage consisted of the utilization of specific primers for the detection of the species A ABSTRACT platys (PCR2). Upon comparison of the re- Anaplasma platys is related to the appear- sults, 18.81% of the studied animals showed ance of inclusion bodies in blood platelets; positive for PCR1. For PCR2, 15.84% of the however, this may be a nonspecific occur- studied animals had a positive result. In the rence as there are nonparasitic inclusion morphological analysis of the inclusion bod- bodies within these figured elements. Aiming ies, 14.85% of the animals showed positive to validate the morphological diagnosis for for A platys. The other inclusion bodies were A platys, 101 dogs were selected due to the considered as nonspecific, therefore nega- appearance of inclusion bodies, indepen- tive. When compared to the morphological dently from suggestive parasites, which analysis, the results of the molecule analysis were submitted to polymerase chain reac- by means of the MacNemar test led to the tion (PCR) carried out in 2 stages. -

Ohio Department of Health, Bureau of Infectious Diseases Disease Name Class A, Requires Immediate Phone Call to Local Health
Ohio Department of Health, Bureau of Infectious Diseases Reporting specifics for select diseases reportable by ELR Class A, requires immediate phone Susceptibilities specimen type Reportable test name (can change if Disease Name other specifics+ call to local health required* specifics~ state/federal case definition or department reporting requirements change) Culture independent diagnostic tests' (CIDT), like BioFire panel or BD MAX, E. histolytica Stain specimen = stool, bile results should be sent as E. histolytica DNA fluid, duodenal fluid, 260373001^DETECTED^SCT with E. histolytica Antigen Amebiasis (Entamoeba histolytica) No No tissue large intestine, disease/organism-specific DNA LOINC E. histolytica Antibody tissue small intestine codes OR a generic CIDT-LOINC code E. histolytica IgM with organism-specific DNA SNOMED E. histolytica IgG codes E. histolytica Total Antibody Ova and Parasite Anthrax Antibody Anthrax Antigen Anthrax EITB Acute Anthrax EITB Convalescent Anthrax Yes No Culture ELISA PCR Stain/microscopy Stain/spore ID Eastern Equine Encephalitis virus Antibody Eastern Equine Encephalitis virus IgG Antibody Eastern Equine Encephalitis virus IgM Arboviral neuroinvasive and non- Eastern Equine Encephalitis virus RNA neuroinvasive disease: Eastern equine California serogroup virus Antibody encephalitis virus disease; LaCrosse Equivocal results are accepted for all California serogroup virus IgG Antibody virus disease (other California arborviral diseases; California serogroup virus IgM Antibody specimen = blood, serum, serogroup -

Gene Gain and Loss Events in Rickettsia and Orientia Species Kalliopi Georgiades1,2, Vicky Merhej1, Khalid El Karkouri1, Didier Raoult1, Pierre Pontarotti2*
Georgiades et al. Biology Direct 2011, 6:6 http://www.biology-direct.com/content/6/1/6 RESEARCH Open Access Gene gain and loss events in Rickettsia and Orientia species Kalliopi Georgiades1,2, Vicky Merhej1, Khalid El Karkouri1, Didier Raoult1, Pierre Pontarotti2* Abstract Background: Genome degradation is an ongoing process in all members of the Rickettsiales order, which makes these bacterial species an excellent model for studying reductive evolution through interspecies variation in genome size and gene content. In this study, we evaluated the degree to which gene loss shaped the content of some Rickettsiales genomes. We shed light on the role played by horizontal gene transfers in the genome evolution of Rickettsiales. Results: Our phylogenomic tree, based on whole-genome content, presented a topology distinct from that of the whole core gene concatenated phylogenetic tree, suggesting that the gene repertoires involved have different evolutionary histories. Indeed, we present evidence for 3 possible horizontal gene transfer events from various organisms to Orientia and 6 to Rickettsia spp., while we also identified 3 possible horizontal gene transfer events from Rickettsia and Orientia to other bacteria. We found 17 putative genes in Rickettsia spp. that are probably the result of de novo gene creation; 2 of these genes appear to be functional. On the basis of these results, we were able to reconstruct the gene repertoires of “proto-Rickettsiales” and “proto-Rickettsiaceae”, which correspond to the ancestors of Rickettsiales and Rickettsiaceae, respectively. Finally, we found that 2,135 genes were lost during the evolution of the Rickettsiaceae to an intracellular lifestyle. Conclusions: Our phylogenetic analysis allowed us to track the gene gain and loss events occurring in bacterial genomes during their evolution from a free-living to an intracellular lifestyle. -

Appendix B the Following List Represents 29 Zoonotic/Anthroponotic Pathogens Denoted As Grey-Zone Pathogens
Appendix B The following list represents 29 zoonotic/anthroponotic pathogens denoted as Grey-Zone Pathogens. These represent any pathogen where there is some evidence the dog is involved in transmission, maintenance or detection of the pathogen, but current research has not definitively proven the dog’s role at the time of this study. There were no sapronoses in this group. Of these pathogens, those that have been reported in Canada are marked with a 1. Those pathogens that have the potential to occur in Canada but have not yet been reported are marked with a 2. Bacteria Protozoa Acinetobacter baumannii 1 Babesia canis canis Borrelia turicatae 1 Babesia canis rossi Campylobacter gracilis 1 Babesia canis vogeli 1 Campylobacter lari 1 Blastocystis hominis 1 Helicobacter bizzozeronii 2 Blastocystis spp. 1 Mycoplasma canis 2 Cryptosporidium parvum 1 Mycoplasma maculosum 2 Leishmania donovani 1 Rhodococcus equi 1 Staphylococcus schleiferi coagulans Rickettsia Anaplasma platys 1 Fungi Ehrlichia chaffeensis 2 Encephalitozoon cuniculi 1 Ehrlichia ewingii 2 Encephalitozoon intestinalis 2 Enterocytozoon bieneusi 2 Viruses Reovirus MRV (Mammalian Reovirus) serotype 1 1 Helminths Reovirus MRV (Mammalian Reovirus) serotype 2 1 Ascaris lumbricoides 1 Reovirus MRV (Mammalian Reovirus) serotype 3 1 Trichinella spiralis 1 West Nile virus1 Trichuris vulpis 1 Only a portion of the information obtained in this study is presented here. Please contact the authors for additional reference material on why a pathogen was or was not included in a particular step. Appendix C The following list represents 74 zoonotic/sapronotic pathogens where the dog is involved in transmission, maintenance, or detection of the pathogen and the pathogen has been reported to have historically occurred in Canada, however, Canadian canine-specific reports are lacking (Tier 2). -

Molecular Detection and Identification of Rickettsiales Pathogens in Dog Ticks from Costa Rica
Accepted Manuscript Title: Molecular detection and identification of Rickettsiales pathogens in dog ticks from Costa Rica Author: Liliana Campos-Calderon´ Leyda Abrego-S´ anchez´ Antony Solorzano-Morales´ Alberto Alberti Gessica Tore Rosanna Zobba Ana E. Jimenez-Rocha´ Gaby Dolz PII: S1877-959X(16)30120-0 DOI: http://dx.doi.org/doi:10.1016/j.ttbdis.2016.07.015 Reference: TTBDIS 700 To appear in: Received date: 29-2-2016 Revised date: 1-7-2016 Accepted date: 24-7-2016 Please cite this article as: Campos-Calderon,´ Liliana, Abrego-S´ anchez,´ Leyda, Solorzano-Morales,´ Antony, Alberti, Alberto, Tore, Gessica, Zobba, Rosanna, Jimenez-Rocha,´ Ana E., Dolz, Gaby, Molecular detection and identification of Rickettsiales pathogens in dog ticks from Costa Rica.Ticks and Tick-borne Diseases http://dx.doi.org/10.1016/j.ttbdis.2016.07.015 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Molecular detection and identification of Rickettsiales pathogens in dog ticks from Costa Rica Liliana Campos-Calderóna, Leyda Ábrego-Sánchezb, Antony Solórzano- Moralesa, Alberto Albertic, Gessica Torec, Rosanna Zobbac, Ana E. Jiménez- Rochaa, Gaby Dolza,b,* aEscuela de Medicina Veterinaria, Universidad Nacional, Campus Benjamín Núñez, Barreal de Heredia, Costa Rica ([email protected], [email protected], [email protected]). -

High Prevalence of Rickettsia Africae Variants in Amblyomma Variegatum Ticks from Domestic Mammals in Rural Western Kenya: Implications for Human Health
n Maina, A. N. et al. (2014) High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector-Borne and Zoonotic Diseases, 14 (10). pp. 693-702. ISSN 1530-3667 Copyright © 2014 The Authors http://eprints.gla.ac.uk/99480/ Deposited on: 17 November 2014 Enlighten – Research publications by members of the University of Glasgow http://eprints.gla.ac.uk VECTOR-BORNE AND ZOONOTIC DISEASES Volume 14, Number 10, 2014 ORIGINAL ARTICLES ª Mary Ann Liebert, Inc. DOI: 10.1089/vbz.2014.1578 High Prevalence of Rickettsia africae Variants in Amblyomma variegatum Ticks from Domestic Mammals in Rural Western Kenya: Implications for Human Health Alice N. Maina,1–3 Ju Jiang,3 Sylvia A. Omulo,2 Sally J. Cutler,4 Fredrick Ade,2 Eric Ogola,2 Daniel R. Feikin,5 M. Kariuki Njenga,5 Sarah Cleaveland,6 Solomon Mpoke,1,2 Zipporah Ng’ang’a,1 Robert F. Breiman,5 Darryn L. Knobel,2,7 and Allen L. Richards3 Abstract Tick-borne spotted fever group (SFG) rickettsioses are emerging human diseases caused by obligate intracel- lular Gram-negative bacteria of the genus Rickettsia. Despite being important causes of systemic febrile illnesses in travelers returning from sub-Saharan Africa, little is known about the reservoir hosts of these pathogens. We conducted surveys for rickettsiae in domestic animals and ticks in a rural setting in western Kenya. Of the 100 serum specimens tested from each species of domestic ruminant 43% of goats, 23% of sheep, and 1% of cattle had immunoglobulin G (IgG) antibodies to the SFG rickettsiae. -

Molecular Detection of Tick-Borne Pathogen Diversities in Ticks From
ORIGINAL RESEARCH published: 01 June 2017 doi: 10.3389/fvets.2017.00073 Molecular Detection of Tick-Borne Pathogen Diversities in Ticks from Livestock and Reptiles along the Shores and Adjacent Islands of Lake Victoria and Lake Baringo, Kenya David Omondi1,2,3, Daniel K. Masiga1, Burtram C. Fielding 2, Edward Kariuki 4, Yvonne Ukamaka Ajamma1,5, Micky M. Mwamuye1, Daniel O. Ouso1,5 and Jandouwe Villinger 1* 1International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya, 2 University of Western Cape, Bellville, South Africa, 3 Egerton University, Egerton, Kenya, 4 Kenya Wildlife Service, Nairobi, Kenya, 5 Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya Although diverse tick-borne pathogens (TBPs) are endemic to East Africa, with recog- nized impact on human and livestock health, their diversity and specific interactions with Edited by: tick and vertebrate host species remain poorly understood in the region. In particular, Dirk Werling, the role of reptiles in TBP epidemiology remains unknown, despite having been impli- Royal Veterinary College, UK cated with TBPs of livestock among exported tortoises and lizards. Understanding TBP Reviewed by: Timothy Connelley, ecologies, and the potential role of common reptiles, is critical for the development of University of Edinburgh, UK targeted transmission control strategies for these neglected tropical disease agents. Abdul Jabbar, University of Melbourne, Australia During the wet months (April–May; October–December) of 2012–2013, we surveyed Ria Ghai, TBP diversity among 4,126 ticks parasitizing livestock and reptiles at homesteads along Emory University, USA the shores and islands of Lake Baringo and Lake Victoria in Kenya, regions endemic *Correspondence: to diverse neglected tick-borne diseases. -

During Flowering Stage and Their Plant Growth Promoting Traits. D Sachdev, V Agarwal, P Verma, Y Shouche, P Dhakephalkar, B Chopade
The Internet Journal of Microbiology ISPUB.COM Volume 7 Number 2 Assessment of microbial biota associated with rhizosphere of wheat (Triticum aestivum) during flowering stage and their plant growth promoting traits. D Sachdev, V Agarwal, P Verma, Y Shouche, P Dhakephalkar, B Chopade Citation D Sachdev, V Agarwal, P Verma, Y Shouche, P Dhakephalkar, B Chopade. Assessment of microbial biota associated with rhizosphere of wheat (Triticum aestivum) during flowering stage and their plant growth promoting traits.. The Internet Journal of Microbiology. 2008 Volume 7 Number 2. Abstract Microbial biota associated with wheat rhizosphere during flowering-stage and their plant growth promoting traits was investigated. 16S rRNA gene sequencing was performed of the isolates, which were obtained on selective media. Isolates belonged to: alpha-proteobacteria, beta-proteobacteria, gamma-proteobacteria; Actinobacteria; Bacteroidetes and Firmicutes. Bacillus was the most dominant genus (34.7%) followed by Pseudomonas (14.4%). Among diazotrophs, Arthrobacter sp. (n=3), Cupriavidus sp. (n=3) and Stenotrophomonas sp. (n=4) occurred more frequently. Most of the isolates produced indole acetic acid. Ac. baumannii , Ps. lini, Ser. marcescens, C. respiraculi and Ag.tumfaciens solubilized phosphate. Two Acinetobacter strains, five Pseudomonas srains and four Bacillus strains produced siderophore. Strains of Ps. aeruginosa, Ps. lini and Ps. thivervalensis exhibited in vitro fungal growth inhibition. Arthrobacter globiformis Y2S3 exhibited indole acetic acid production, siderophore production and antifungal activity. Plant growth promoting traits of these rhizobacteria indicated beneficial relationship between rhizobacteria and wheat plants. Several of the strains could be further developed as an effective bio-inoculant. INTRODUCTION plant growth (Compant et al. 2005; Padalalu and Chopade Rhizosphere soil is a “hot-spot” for microbial growth and 2006). -

Exploration of Tick-Borne Pathogens and Microbiota of Dog Ticks Collected at Potchefstroom Animal Welfare Society
Exploration of tick-borne pathogens and microbiota of dog ticks collected at Potchefstroom Animal Welfare Society C Van Wyk orcid.org 0000-0002-5971-4396 Dissertation submitted in fulfilment of the requirements for the degree Master of Science in Environmental Sciences at the North-West University Supervisor: Prof MMO Thekisoe Co-supervisor: Ms K Mtshali Graduation May 2019 24263524 DEDICATION This thesis is dedicated to the late Nettie Coetzee. For her inspiration and lessons to overcome any obstacle that life may present. God called home another angel we all love and miss you. “We are the scientists, trying to make sense of the stars inside us.” -Christopher Poindexter i ACKNOWLEDGEMENTS My sincerest appreciation goes out to my supervisor, Prof. Oriel M.M. Thekisoe, for his support, motivation, guidance, and insightfulness during the duration of this project and been there every step of the way. I would also like to thank my co-supervisor, Ms. Khethiwe Mtshali, for her patience and insightfulness towards the corrections of this thesis. I would like to thank Dr. Stalone Terera and the staff members at PAWS for their aid towards the collection of tick specimens. For the sequencing on the Illumina MiSeq platform and metagenomic data analysis I would like to thank Dr. Moeti O. Taioe, Dr. Charlotte M.S. Mienie, Dr. Danie C. La Grange, and Dr. Marlin J. Mert. I would like to thank the National Research Foundation (NRF) for their financial support by awarding me the S&F- Innovation Masters Scholarship and the North-West University (NWU) for the use of their laboratories.