Exploration of Tick-Borne Pathogens and Microbiota of Dog Ticks Collected at Potchefstroom Animal Welfare Society
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Community Analysis of Microbial Sharing and Specialization in A
Downloaded from http://rspb.royalsocietypublishing.org/ on March 15, 2017 Community analysis of microbial sharing rspb.royalsocietypublishing.org and specialization in a Costa Rican ant–plant–hemipteran symbiosis Elizabeth G. Pringle1,2 and Corrie S. Moreau3 Research 1Department of Biology, Program in Ecology, Evolution, and Conservation Biology, University of Nevada, Cite this article: Pringle EG, Moreau CS. 2017 Reno, NV 89557, USA 2Michigan Society of Fellows, University of Michigan, Ann Arbor, MI 48109, USA Community analysis of microbial sharing and 3Department of Science and Education, Field Museum of Natural History, 1400 South Lake Shore Drive, specialization in a Costa Rican ant–plant– Chicago, IL 60605, USA hemipteran symbiosis. Proc. R. Soc. B 284: EGP, 0000-0002-4398-9272 20162770. http://dx.doi.org/10.1098/rspb.2016.2770 Ants have long been renowned for their intimate mutualisms with tropho- bionts and plants and more recently appreciated for their widespread and diverse interactions with microbes. An open question in symbiosis research is the extent to which environmental influence, including the exchange of Received: 14 December 2016 microbes between interacting macroorganisms, affects the composition and Accepted: 17 January 2017 function of symbiotic microbial communities. Here we approached this ques- tion by investigating symbiosis within symbiosis. Ant–plant–hemipteran symbioses are hallmarks of tropical ecosystems that produce persistent close contact among the macroorganism partners, which then have substantial opportunity to exchange symbiotic microbes. We used metabarcoding and Subject Category: quantitative PCR to examine community structure of both bacteria and Ecology fungi in a Neotropical ant–plant–scale-insect symbiosis. Both phloem-feed- ing scale insects and honeydew-feeding ants make use of microbial Subject Areas: symbionts to subsist on phloem-derived diets of suboptimal nutritional qual- ecology, evolution, microbiology ity. -

Inactivation of CRISPR-Cas Systems by Anti-CRISPR Proteins in Diverse Bacterial Species April Pawluk1, Raymond H.J
LETTERS PUBLISHED: 13 JUNE 2016 | ARTICLE NUMBER: 16085 | DOI: 10.1038/NMICROBIOL.2016.85 Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species April Pawluk1, Raymond H.J. Staals2, Corinda Taylor2, Bridget N.J. Watson2, Senjuti Saha3, Peter C. Fineran2, Karen L. Maxwell4* and Alan R. Davidson1,3* CRISPR-Cas systems provide sequence-specific adaptive immu- MGE-encoded mechanisms that inhibit CRISPR-Cas systems. In nity against foreign nucleic acids1,2. They are present in approxi- support of this hypothesis, phages infecting Pseudomonas aeruginosa mately half of all sequenced prokaryotes3 and are expected to were found to encode diverse families of proteins that inhibit constitute a major barrier to horizontal gene transfer. We pre- the CRISPR-Cas systems of their host through several distinct viously described nine distinct families of proteins encoded in mechanisms4,5,17,18. However, homologues of these anti-CRISPR Pseudomonas phage genomes that inhibit CRISPR-Cas function4,5. proteins were found only within the Pseudomonas genus. Here, We have developed a bioinformatic approach that enabled us to we describe a bioinformatic approach that allowed us to identify discover additional anti-CRISPR proteins encoded in phages five novel families of functional anti-CRISPR proteins encoded in and other mobile genetic elements of diverse bacterial phages and other putative MGEs in species spanning the diversity species. We show that five previously undiscovered families of Proteobacteria. of anti-CRISPRs inhibit the type I-F CRISPR-Cas systems of The nine previously characterized anti-CRISPR protein families both Pseudomonas aeruginosa and Pectobacterium atrosepticum, possess no common sequence motifs, so we used genomic context to and a dual specificity anti-CRISPR inactivates both type I-F search for novel anti-CRISPR genes. -

(Batch Learning Self-Organizing Maps), to the Microbiome Analysis of Ticks
Title A novel approach, based on BLSOMs (Batch Learning Self-Organizing Maps), to the microbiome analysis of ticks Nakao, Ryo; Abe, Takashi; Nijhof, Ard M; Yamamoto, Seigo; Jongejan, Frans; Ikemura, Toshimichi; Sugimoto, Author(s) Chihiro The ISME Journal, 7(5), 1003-1015 Citation https://doi.org/10.1038/ismej.2012.171 Issue Date 2013-03 Doc URL http://hdl.handle.net/2115/53167 Type article (author version) File Information ISME_Nakao.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP A novel approach, based on BLSOMs (Batch Learning Self-Organizing Maps), to the microbiome analysis of ticks Ryo Nakao1,a, Takashi Abe2,3,a, Ard M. Nijhof4, Seigo Yamamoto5, Frans Jongejan6,7, Toshimichi Ikemura2, Chihiro Sugimoto1 1Division of Collaboration and Education, Research Center for Zoonosis Control, Hokkaido University, Kita-20, Nishi-10, Kita-ku, Sapporo, Hokkaido 001-0020, Japan 2Nagahama Institute of Bio-Science and Technology, Nagahama, Shiga 526-0829, Japan 3Graduate School of Science & Technology, Niigata University, 8050, Igarashi 2-no-cho, Nishi- ku, Niigata 950-2181, Japan 4Institute for Parasitology and Tropical Veterinary Medicine, Freie Universität Berlin, Königsweg 67, 14163 Berlin, Germany 5Miyazaki Prefectural Institute for Public Health and Environment, 2-3-2 Gakuen Kibanadai Nishi, Miyazaki 889-2155, Japan 6Utrecht Centre for Tick-borne Diseases (UCTD), Department of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 1, 3584 CL Utrecht, The Netherlands 7Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, 0110 Onderstepoort, South Africa aThese authors contributed equally to this work. Keywords: BLSOMs/emerging diseases/metagenomics/microbiomes/symbionts/ticks Running title: Tick microbiomes revealed by BLSOMs Subject category: Microbe-microbe and microbe-host interactions Abstract Ticks transmit a variety of viral, bacterial and protozoal pathogens, which are often zoonotic. -

Morbidity and Mortality Weekly Report Weekly March 20, 2009 / Vol
Morbidity and Mortality Weekly Report www.cdc.gov/mmwr Weekly March 20, 2009 / Vol. 58 / No. 10 Trends in Tuberculosis — World TB Day — March 24, 2009 United States, 2008 World TB Day is observed each year on March 24 to commemorate the date in 1882 when Dr. Robert Koch In 2008, a total of 12,898 incident tuberculosis (TB) cases announced the discovery of Mycobacterium tuberculosis, the were reported in the United States; the TB rate declined 3.8% bacterium that causes tuberculosis (TB). Worldwide, TB from 2007 to 4.2 cases per 100,000 population, the lowest remains one of the leading causes of death from infectious rate recorded since national reporting began in 1953. This disease. An estimated 2 billion persons are infected with report summarizes provisional 2008 data from the National M. tuberculosis (1). In 2006, approximately 9.2 million TB Surveillance System and describes trends since 1993. persons became ill from TB, and 1.7 million died from Despite this overall improvement, progress has slowed in the disease (1). World TB Day provides an opportunity recent years; the average annual percentage decline in the TB for TB programs, nongovernmental organizations, and rate decreased from 7.3% per year during 1993–2000 to 3.8% other partners to describe problems and solutions related during 2000–2008.* Foreign-born persons and racial/ethnic to the TB pandemic and to support worldwide TB minorities continued to bear a disproportionate burden of TB control efforts. The U.S. theme for this year’s observance disease in the United States. In 2008, the TB rate in foreign- is Partnerships for TB Elimination. -

Babela Massiliensis, a Representative of a Widespread Bacterial
Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, Samia Benamar, Didier Raoult, Eugene V. Koonin, Bernard La Scola To cite this version: Isabelle Pagnier, Natalya Yutin, Olivier Croce, Kira S Makarova, Yuri I Wolf, et al.. Babela mas- siliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae. Biology Direct, BioMed Central, 2015, 10 (13), 10.1186/s13062-015-0043-z. hal-01217089 HAL Id: hal-01217089 https://hal-amu.archives-ouvertes.fr/hal-01217089 Submitted on 19 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Pagnier et al. Biology Direct (2015) 10:13 DOI 10.1186/s13062-015-0043-z RESEARCH Open Access Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae Isabelle Pagnier1, Natalya Yutin2, Olivier Croce1, Kira S Makarova2, Yuri I Wolf2, Samia Benamar1, Didier Raoult1, Eugene V Koonin2 and Bernard La Scola1* Abstract Background: Only a small fraction of bacteria and archaea that are identifiable by metagenomics can be grown on standard media. -

2012 Case Definitions Infectious Disease
Arizona Department of Health Services Case Definitions for Reportable Communicable Morbidities 2012 TABLE OF CONTENTS Definition of Terms Used in Case Classification .......................................................................................................... 6 Definition of Bi-national Case ............................................................................................................................................. 7 ------------------------------------------------------------------------------------------------------- ............................................... 7 AMEBIASIS ............................................................................................................................................................................. 8 ANTHRAX (β) ......................................................................................................................................................................... 9 ASEPTIC MENINGITIS (viral) ......................................................................................................................................... 11 BASIDIOBOLOMYCOSIS ................................................................................................................................................. 12 BOTULISM, FOODBORNE (β) ....................................................................................................................................... 13 BOTULISM, INFANT (β) ................................................................................................................................................... -

Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate Or Sertraline, to Fight Against Helicobacter Pylori Lawn Biofilm
International Journal of Molecular Sciences Article Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm Paweł Krzy˙zek 1,* , Gra˙zynaGo´sciniak 1 , Karol Fijałkowski 2 , Paweł Migdał 3 , Mariusz Dziadas 4 , Artur Owczarek 5 , Joanna Czajkowska 6, Olga Aniołek 7 and Adam Junka 8 1 Department of Microbiology, Faculty of Medicine, Wroclaw Medical University, 50-368 Wroclaw, Poland; [email protected] 2 Department of Immunology, Microbiology and Physiological Chemistry, Faculty of Biotechnology and Animal Husbandry, West Pomeranian University of Technology in Szczecin, 70-311 Szczecin, Poland; karol.fi[email protected] 3 Department of Environment, Hygiene and Animal Welfare, Wroclaw University of Environmental and Life Sciences, 51-630 Wroclaw, Poland; [email protected] 4 Faculty of Chemistry, University of Wroclaw, 50-353 Wroclaw, Poland; [email protected] 5 Department of Drug Form Technology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] 6 Laboratory of Microbiology, Polish Center for Technology Development PORT, 54-066 Wroclaw, Poland; [email protected] 7 Faculty of Medicine, Lazarski University, 02-662 Warsaw, Poland; [email protected] 8 Department of Pharmaceutical Microbiology and Parasitology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] * Correspondence: [email protected] Received: 23 November 2020; Accepted: 11 December 2020; Published: 14 December 2020 Abstract: Helicobacter pylori is a bacterium known mainly of its ability to cause persistent inflammations of the human stomach, resulting in peptic ulcer diseases and gastric cancers. Continuous exposure of this bacterium to antibiotics has resulted in high detection of multidrug-resistant strains and difficulties in obtaining a therapeutic effect. -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

Downloaded 13 April 2017); Using Diamond
bioRxiv preprint doi: https://doi.org/10.1101/347021; this version posted June 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 2 3 4 5 Re-evaluating the salty divide: phylogenetic specificity of 6 transitions between marine and freshwater systems 7 8 9 10 Sara F. Pavera, Daniel J. Muratorea, Ryan J. Newtonb, Maureen L. Colemana# 11 a 12 Department of the Geophysical Sciences, University of Chicago, Chicago, Illinois, USA 13 b School of Freshwater Sciences, University of Wisconsin Milwaukee, Milwaukee, Wisconsin, USA 14 15 Running title: Marine-freshwater phylogenetic specificity 16 17 #Address correspondence to Maureen Coleman, [email protected] 18 bioRxiv preprint doi: https://doi.org/10.1101/347021; this version posted June 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 19 Abstract 20 Marine and freshwater microbial communities are phylogenetically distinct and transitions 21 between habitat types are thought to be infrequent. We compared the phylogenetic diversity of 22 marine and freshwater microorganisms and identified specific lineages exhibiting notably high or 23 low similarity between marine and freshwater ecosystems using a meta-analysis of 16S rRNA 24 gene tag-sequencing datasets. As expected, marine and freshwater microbial communities 25 differed in the relative abundance of major phyla and contained habitat-specific lineages; at the 26 same time, however, many shared taxa were observed in both environments. 27 Betaproteobacteria and Alphaproteobacteria sequences had the highest similarity between 28 marine and freshwater sample pairs. -
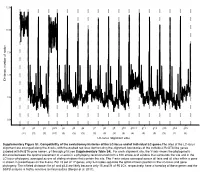
LC-Locus Alignment Sites Distance, Number of Nodes Supplementary
12.5 10.0 7.5 5.0 Distance, number of nodes 2.5 0.0 g1 g2 g3 g3.5 g4 g5 g6 g7 g8 g9 g10 g10.1 g11 g12 g13 g14 g15 (11) (3) (3) (10) (3) (3) (3) (3) (3) (3) (3) (4) (3) (3) (3) (2) (3) LC-locus alignment sites Supplementary Figure S1. Compatibility of the evolutionary histories of the LC-locus and of individual LC genes.The sites of the LC-locus alignment are arranged along the X-axis, with the dashed red lines demarcating the alignment boundaries of the individual RcGTA-like genes (labeled with RcGTA gene names, g1 through g15; see Supplementary Table S4). For each alignment site, the Y-axis shows the phylogenetic distance between the optimal placement of a taxon in a phylogeny reconstructed from a 100 amino-acid window that surrounds the site and in the LC-locus phylogeny, averaged across all sliding windows that contain the site. The Y-axis values averaged across all taxa and all sites within a gene is shown in parentheses on the X-axis. For 15 out of 17 genes, only 2-4 nodes separate the optimal taxon position in the LC-locus and gene phylogeny. The inflated distances for g1 and g3.5 are likely because only 15 and 21 of 95 LCs, respectively, have a homolog of these genes and the SSPB analysis is highly sensitive to missing data (Berger et al. 2011). a. Bacteria Unassigned Thermotogae Tenericutes Synergistetes Spirochaetes Proteobacteria ylum Planctomycetes h p Firmicutes Deferribacteres Cyanobacteria Chloroflexi Bacteroidetes Actinobacteria Acidobacteria 1(11,750) 2(1,750) 3(2,538) 4(168) 5(51) 6(54) 7(43) 8(32) 9(26) 10(40) 11(33) 12(198) 13(173) 14(101) 15(98) 16(43) 17(114) Number of rcc01682−rcc01698 homologs in a cluster b. -

Ehrlichia Ewingii Sp. Nov., the Etiologic Agent of Canine Granulocytic Ehrlichiosis
INTERNATIONAL JOURNAL OF SYSTEMATICBACTERIOLOGY, Apr. 1992, p. 299-302 Vol. 42, No. 2 0020-7713/92/020299-04$02.00/0 Copyright 0 1992, International Union of Microbiological Societies NOTES Ehrlichia ewingii sp. nov., the Etiologic Agent of Canine Granulocytic Ehrlichiosis BURT E. ANDERSON,l* CRAIG E. GREENE,2 DANA C. JONES,l AND JACQUELINE E. DAWSON’ viral and Rickettsial Zoonoses Branch, Division of viral and Rickettsial Diseases, National Center for Infectious Diseases, Centers for Disease Control, Atlanta, Georgia 30333, and Department of Small Animal Medicine, College of Veterinaly Medicine, University of Georgia, Athens, Georgia 306022 The 16s rRNA gene was amplified, cloned, and sequenced from the blood of two dogs that were experimentally infected with the etiologic agent of canine granulocytic ehrlichiosis. The 16s rRNA sequence was found to be unique when it was compared with the sequences of other members of the genus Ehrlichia. The most closely related species were Ehrlichia canis (98.0% related) and the human ehrlichiosis agent (Ehrlichia chafeensis) (98.1% related); all other species in the genus were found to be phylogenetically much more distant. Our results, coupled with previous serologic data, provide conclusive evidence that the canine granulocytic ehrlichiosis agent is a new species of the genus Ehrlichia that is related to, but is distinct from, E. canis and all other members of the genus. We propose the name Ehrlichia ewingii sp. nov.; the Stillwater strain is the type strain. Ehrlichia canis, the type species of the genus Ehrlichia, human ehrlichiosis (Ehrlichia chafeensis) (1) is discussed was first described by Donatien and Lestoquard in 1935 (7). -

(Chiroptera: Vespertilionidae) and the Bat Soft Tick Argas Vespe
Zhao et al. Parasites Vectors (2020) 13:10 https://doi.org/10.1186/s13071-020-3885-x Parasites & Vectors SHORT REPORT Open Access Rickettsiae in the common pipistrelle Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) and the bat soft tick Argas vespertilionis (Ixodida: Argasidae) Shuo Zhao1†, Meihua Yang2†, Gang Liu1†, Sándor Hornok3, Shanshan Zhao1, Chunli Sang1, Wenbo Tan1 and Yuanzhi Wang1* Abstract Background: Increasing molecular evidence supports that bats and/or their ectoparasites may harbor vector-borne bacteria, such as bartonellae and borreliae. However, the simultaneous occurrence of rickettsiae in bats and bat ticks has been poorly studied. Methods: In this study, 54 bat carcasses and their infesting soft ticks (n 67) were collected in Shihezi City, north- western China. The heart, liver, spleen, lung, kidney, small intestine and large= intestine of bats were dissected, followed by DNA extraction. Soft ticks were identifed both morphologically and molecularly. All samples were examined for the presence of rickettsiae by amplifying four genetic markers (17-kDa, gltA, ompA and ompB). Results: All bats were identifed as Pipistrellus pipistrellus, and their ticks as Argas vespertilionis. Molecular analyses showed that DNA of Rickettsia parkeri, R. lusitaniae, R. slovaca and R. raoultii was present in bat organs/tissues. In addition, nine of the 67 bat soft ticks (13.43%) were positive for R. raoultii (n 5) and R. rickettsii (n 4). In the phylo- genetic analysis, these bat-associated rickettsiae clustered together with conspecifc= sequences reported= from other host and tick species, confrming the above results. Conclusions: To the best of our knowledge, DNA of R. parkeri, R. slovaca and R.