To Graze Or Not to Graze: That Is the Question
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Verslag Algemene Ledenavond Woensdag 11 Maart 2015.Pdf
Verslag Algemene ledenavond woensdag 11 maart 2015 Voorzitter Piet Tinus van der Wal opent de vergadering en heet iedereen welkom. Na een korte algemene ledenvergadering begint dr. Albert Buursma aan de lezing WADDEN IN BEWEGING Lezing: Het gaat vooral over de geschiedenis van de Oostelijke Waddenzee. Het ontstaan van Middelstum heeft zeker te maken door de vorming van de Wadden. Albert Buursma is al 10 jaar bezig met de geschiedenis van Rottemerplaat en Rottemeroog. Onder Vlieland is heel diep een Vulkanische pijp waar genomen. Men heeft zelfs resten van Dinosaurussen gevonden. Ooit was de Noordzee droog. Men kon zo’n 10.000 tot 5000 jaar geleden naar Engeland lopen. Bij de Doggersbank zijn resten van botten en schedels gevonden. Om het wegslaan van de duinen bij Ameland , Vlieland en Terschelling tegen te gaan is er zand uit de zee langs de kustlijn aangebracht. Soms wordt daarin iets gevonden, zoals een vuursteen, een Romeinse amfora en ander Romeins aardewerk. Bij Zoutkamp heeft men munten van soldij gevonden, Het zijn eigenlijk de tussen kunst en kitsch gevonden voorwerpen op de kusteilanden, afkomstig van scheepswrakken. Vanaf 1000 jaar v. Chr. zijn de wierden en terpen ontstaan. Het gebied hier was een kwelderlandschap waarin ook Middelstum lag. De eerste wierden met hun boerderijen lagen op zo’n 3 à 4 km van elkaar vandaan. De Halligen vormen een groep eilandjes in het noordelijke deel van de Duitse Waddenzee. De eilanden hebben bij elkaar niet meer dan driehonderd inwoners. Groot Zeewijk in de Noordpolder is rond 1800 na Chr. ingedijkt. Tot zolang was het een buitengebied, een Hallig. -

Ausflugsfahrten 2021
RegelnCOVID-19 im Innenteil Fahrkarten Online unter wattenmeerfahrten.de oder im Vorverkauf: Föhr-Amrumer Reisebüro (Wyk), Tourist-Informationen (Wyk, Nieblum, Utersum) List Sie können Ihre Fahrkarte auch am Schiff vor der Abfahrt erwerben, solange Plätze frei sind. Ausflugsfahrten Hallig Hooge Große Halligmeer-Kreuzfahrt Seetierfang MS Hauke Haien stellt sich vor Alle Schiffsabfahrts- und Ankunftszeiten gelten nur bei normalen Wind-, Wasser- und Sichtverhältnissen sowie genügender Beteiligung. Irrtum und Änderungen vorbehal- ten. Ankunftszeiten können auf Grund der Tide variieren. Es gelten die Beförderungs- Erwachsene Kinder (4-14 J) Familien* Erwachsene Kinder (4-14 J) Familien* Erwachsene Kinder (4-14 J) Familien* bedingungen der Halligreederei MS Hauke Haien. Wir, die Familie Diedrichsen, betreiben das Schiff seit 1988 2021 35 € 15 € 95 € 35 € 15 € 95 € 30 € 15 € 80 € und unser Heimathafen ist Hallig Hooge. Den Namen „Hau- ke Haien“ erhielt das Schiff nach der Hauptfigur aus Theodor Inkl. Seetierfang & Seehundsbänke (tideabhängig) Auf Hallig Gröde (1 Std. Landgang) · Seehundsbänke (tideabh.) Auf diesen Touren zeigen wir Ihnen die Unterwasserwelt. In der Für besondere Anlässe können Sie unser Schiff Wir wollen Sie auf Seereise zur Hallig Hooge mitnehmen. Am An- Unser Kurs geht ins östliche Wattenmeer vorbei an den Halligen Nähe der Wyker Küste wird ein Schleppnetz ausgeworfen und Storms Novelle „Der Schimmelreiter“. Unser Schiff wurde 1960 ab Wyk auf Föhr (alte Mole) leger können Fahrräder oder Kutschen gebucht werden, oder Sie Langeneß , Hooge, Oland, Gröde, Habel, Hamburger Hallig, Nord- der Seetierfang an Bord vom Kapitän oder der gebürtigen Nord- als erste Halligfähre von „Kapitän August Jakobs“ mit dem Na- auch chartern. Sprechen Sie uns gerneNiebüll an. -

Wattenmeer Für Alle
BARRIEREFREIE NATURERLEBNISANGEBOTE IM NATIONALPARK Wattenmeer für Alle Nationalpark Wa ttenmeer SCHLESWIG-HOLSTEIN Hinweise zu Covid-19 Alle Änderungen bezüglich eines Lockdowns oder wegen geltender Covid-19-Maßnahmen sind nicht in dieser Broschüre aufgeführt. Bitte kontaktieren Sie in jedem Fall die Anbieterin oder den Anbieter ob Angebote momentan stattfinden und mit welchen Änderungen zu rechnen ist. Bitte informieren Sie sich rechtzeitig auch auf den entsprechenden Internetseiten über aktuelle Änderungen. Alle Kontaktdaten finden Sie in dieser Broschüre auf den entsprechenden Seiten des Angebotes. Kontaktdaten der Nationalparkverwaltung: Infotelefon: 0 48 61 / 96 20 0 E-Mail: [email protected] 2 Inhalt Zu dieser Broschüre �������������������������������������������������������������������������������������������������������������������4 Der Nationalpark Schleswig-Holsteinisches Wattenmeer ...........................................5 Lebensraum Watt �����������������������������������������������������������������������������������������������������������������������7 Nationalpark-Partner ����������������������������������������������������������������������������������������������������������������8 Hinweise zur Anreise mit der Bahn ......................................................................................9 Barrierefreie Angebote auf Sylt .......................................................................................... 10 Barrierefreie Angebote auf Föhr ....................................................................................... -

Proefschrift R. Kats
University of Groningen Common eiders Somateria mollissima in the Netherlands Kats, Romke Kerst Hendrik IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2007 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Kats, R. K. H. (2007). Common eiders Somateria mollissima in the Netherlands: The rise and fall of breeding and wintering populations in relation to stocks of shellfish. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 11-10-2021 Common Eiders Somateria mollissima in the Netherlands: The rise and fall of breeding and wintering populations in relation to the stocks of shellfish The research presented in this thesis was conducted at IMARES on Texel (formerly know as IBN and Alterra-Texel) and supported by Alterra, IMARES, the Animal Ecology Group (part of the Centre for Evolutionary and Ecological Studies of the University of Groningen), SOVON, NIOZ, and NWO. -
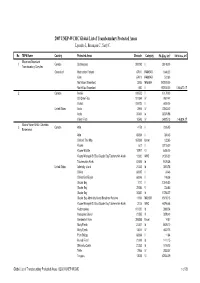
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

No Slide Title
HydrographicHydrographic SocietySociety RenewableRenewable EnergyEnergy Iain Todd Renewables Champion 21 October 2010 THE MOVE TO RENEWABLES • Environmental EUROPE – 2100 (UNCHANGED) THE MOVE TO RENEWABLES • Environmental • Energy security RUSSIAN GAS PIPELINE THE MOVE TO RENEWABLES • Environmental • Energy security • Economic prospects TARGETS • EU has legislated that 20% of all energy to be renewable by 2020 TARGETS • EU has legislated that 20% of all energy to be renewable by 2020 • UK target 15% energy by 2020 TARGETS • EU has legislated that 20% of all energy to be renewable by 2020 • UK target 15% energy by 2020 • Scottish target 20% of energy by 2020 • (31% electricity by 2011, and 50% by 2020) EUROPE – wind resource Full Load Hours/Year EFFECTIVENESS (1) EFFECTIVENESS (2) OFFSHORE PROJECTS – R1 GREAT YARMOUTH GREAT YARMOUTH ROUND 2 BEATRICE FABRICATION BEATRICE • Beatrice project world-leading • Largest (5 MW) • Deepest (45 metres) • First jacket fabrication • Onshore fabrication • Possible extension to 200 turbines (1000 MW) TALISMAN - BEATRICE (1) TALISMAN - BEATRICE (2) TALISMAN - BEATRICE (3) ROUND 3 Wind Power Development Trend EWEA Scenarios (2007); “Reference” equates to EU targets. PIONEERING NORTH SEA INTRODUCING AREG • Created in 2004 • To help transfer oil and gas expertise into renewables agenda • To mutual benefit • Image and awareness of Aberdeen • “Make Aberdeen as famous for renewables as it is for oil and gas” INTRODUCING AREG • Public-private - board of directors • 160 members – public sector, research and development, -

Vestas Wins 42 MW Order for One of the Largest Citizen-Owned Wind
News release from Vestas Central Europe Hamburg, 28 December 2016 Vestas receives 30 MW order and 20-year service contract from citizen-owned wind power plant in northern Germany Vestas has received an order for a repowering project consisting of nine V117-3.45 MW turbines from Bürger-Windpark Lübke-Koog Nord GmbH & Co.KG The firm and unconditional order comprises supply and commissioning of the wind turbines as well as a 20-year full-scope Active Output Management 5000 (AOM5000) service agreement. The wind turbines will be installed at Friedrich-Wilhelm-Lübke-Koog in Schleswig-Holstein, close to the North Sea. The project is a repowering project and replaces thirteen V80-2.0 MW turbines. Wind turbine delivery and commissioning is planned for the third quarter of 2017. Lübke-Koog has a long tradition of citizen-owned wind power plants and is home of the first citizen- owned wind park in Germany, installed in 1991. After the repowering project is completed, 95% of all citizens of Lübke Koog will be participating in the project. “We are very proud of this high participation rate, which creates a strong local support for wind energy. The wind power plant will make an important contribution to regional value creation through tax revenue and donations, supporting local cultural and social activities,” says Arne Möbest, General Manager of Bürger-Windpark Lübke-Koog Nord. “We are working with Vestas since 2004. Vestas has proved to be a strong partner with an excellent service offering and technically reliable wind turbines.” “We are very pleased that our long-term customer, Bürger-Windpark Lübke-Koog Nord GmbH & Co. -

Biodiversity and Ecosystem Processes in an Experimental Island System
Biodiversity and ecosystem processes in an experimental island system Dissertation to obtain the Dr. sc. agr. In the Ph. D. Program for Agricultural Sciences in Göttingen (PAG) At the Faculty of Agricultural Sciences, Georg-August-University Göttingen, Germany Presented by Hagen Andert Born in Görlitz (Germany) Göttingen, September 2017 D 7 1. Referentin/Referent: Prof. Dr. Teja Tscharntke 2. Korreferentin/Korreferent: Prof. Dr. Christoph Scherber Tag der mündlichen Prüfung: 15. November 2017 2 To Darja, Arnt and Lea, and those, who always keep the bright lantern burning in dark nights. 3 Alles Wissen und alle Vermehrung unseres Wissens endet nicht mit einem Schlusspunkt, sondern mit Fragezeichen. [All knowledge and all multiplication of our knowledge does not end with a final point, but with question marks.] Hermann Hesse (1877-1962) 4 Contents CHAPTER 1: .............................................................................................................................. 7 General Introduction .................................................................................................................. 7 GENERAL INTRODUCTION .......................................................................................... 8 STUDY REGION AND EXPERIMENTAL ISLAND SYSTEM ..................................... 9 The German barrier island Spiekeroog .................................................................................. 9 Experimental Islands – the BEFmate project ...................................................................... -

North Sea (Germany) Including Information on the Culicids (Diptera
LüHKEN et at.: 87-95 Studia dipterotogica 1 6 (2009) Heft 1 /2 . ISSN 0945'3954 Mosquito species on the Island of Baltrum in the southern North Sea (Germany) including information on the culicids from the Islands of Langeoog and Mellum (Diptera: Culicioäe) [Die Stechmucken-Arten der Insel Baltrum in der südlichen Nordsee (Deutschland) einschließlich Informationen zu den Culiciden der Inseln Langeoog und Mellum (Diptera: Culicidae)l by RenKe LÜHKEN, E,IIen KIEL, Tammo LIECKWEG and RoIf NIEDRINGHAUS Oldenburg (Germany) Abstract During the summer of 2008, the species composition of mosquitocs (Diptera. Culictdae) rvas studied for three East Frisian lslands in northern German-v. On the Island of Baltrum,4T pools and ditches rvithin a salt marsh and dune complex rr ere sampled rvith sweep nets approximately even' t\\'o ueeks fiom,.\pril to Jull'2008. Adult mosquitoes rverc collected wrth a fixed light trap tiont July' to November 2008. Additionalll/' random samples rvere taken from comparable waterbodies on the islands ofLangeoog and Mellum ber$een July and September 2008. A total ofnrne tara rvere identified . .Anopheles maculipennis complex, Anopheles claviger com- plex,Ochlerotatuscaspius(P.tl.rs. l77l).Ochlerotalusdetitus (Her-lo.w, 1833),Ochlerotatus dorsalls (MercE^- , | 830). Ochterotalils rlslicrs (Ross t, 1790), Culex piplens LruN.q.sus, l7 58, Culex torrentiutll N{.rxrrNt. 1925. and Culiseta annulala (ScumNr, 1776).Five species were recorded for the first time on the Ea-st F'risian Islands.. Ochlerotatus caspius, Oc. detritus. Oc. dorsalis, Oc. r.ttsticus and C.uler lorrenlitol. Four mosquito taxa were recorded for the first time on Baltrum: ,lnopheles nnculipennis contpler, An. -

The Cultural Heritage of the Wadden Sea
The Cultural Heritage of the Wadden Sea 1. Overview Name: Wadden Sea Delimitation: Between the Zeegat van Texel (i.e. Marsdiep, 52° 59´N, 4° 44´E) in the west, and Blåvands Huk in the north-east. On its seaward side it is bordered by the West, East and North Frisian Islands, the Danish Islands of Fanø, Rømø and Mandø and the North Sea. Its landward border is formed by embankments along the Dutch provinces of North- Holland, Friesland and Groningen, the German state of Lower Saxony and southern Denmark and Schleswig-Holstein. Size: Approx. 12,500 square km. Location-map: Borders from west to east the southern mainland-shore of the North Sea in Western Europe. Origin of name: ‘Wad’, ‘watt’ or ‘vad’ meaning a ford or shallow place. This is presumably derives from the fact that it is possible to cross by foot large areas of this sea during the ebb-tides (comparable to Latin vadum, vado, a fordable sea or lake). Relationship/similarities with other cultural entities: Has a direct relationship with the Frisian Islands and the western Danish islands and the coast of the Netherlands, Lower Saxony, Schleswig-Holstein and south Denmark. Characteristic elements and ensembles: The Wadden Sea is a tidal-flat area and as such the largest of its kind in Europe. A tidal-flat area is a relatively wide area (for the most part separated from the open sea – North Sea ̶ by a chain of barrier- islands, the Frisian Islands) which is for the greater part covered by seawater at high tides but uncovered at low tides. -

A Natural History of the Wadden Sea
A natural history of the Wadden Sea Riddled by contingencies Karsten Reise People who are always praising the past And especially the times of faith as best Ought to go back to the middle ages and be burned at the stake as witches and sages. Stevie Smith (1902-1971) Contents 4 Preface by Jens Enemark 50 Chapter 5 Beginning of a new wadden alliance? 6 Contents in a clamshell. A few preliminary comments by the author 60 Chapter 6 How natural is wadden nature? 7 Introduction 70 Chapter 7 What does the future hold 10 Chapter 1 for the Wadden Sea? Why natural history? 80 Conclusions and 22 Chapter 2 recommendations Contingency in natural history What is contingency? 84 The Author / Acknowledgements 34 Chapter 3 On the origin of the Wadden Sea 86 Endnotes 42 Chapter 4 Invited to drown 88 Bibliography Hoofdstuk Preface It is a privilege to write the preface of this booklet by Karsten Contingency is a central notion in Reise’s work and vision. Reise A natural history of the Wadden Sea. Riddled by contingencies. It refers to spaceandtime coincidences and accidental events. It is the fourth booklet in a series that is being published to mark Contingency is almost always involved in natural patterns. The the occasion of the special lectures being held at the symposia natural history of the Wadden Sea displays such contingencies. organised by the Wadden Academy. It cannot be understood as a selfsustaining and resilient system with an inbuilt capability to find a natural balance. Attention to Karsten Reise delivered the keynote address entitled ‘Turning contingency will strengthen realism and promote prudence tides: A natural history of the Wadden Sea’ at the 13th Inter when it comes to projections for the future, Karsten Reise argues. -

Wadlopers Verliezen Favoriete Bestemrning
l"f n {) a :,ffi ttitle Horn Rottrtmcrnlaal ROttUmerOOg ,,v((v",u'H,uu( \ "r Borkum Schiermonnikoog f Zuiderduintjes Ameland Enqelsman- -plaat !: Duitsland Eemshaven @TTOuw:MICHELVAN ELK -$,t: \n ffi'#****ï Expeditie naar Simonszand. Voortaan is de plaat onbereikbaar voor wadlopers FOTOHENK POSTMÁ Wadlopersverliezen favorietebestemrning r Hongerigezee snijdt eilandje Simonszanddootmidden Ineke Noordhoff een eiland waar in 7777 volgens het der."Die laatstekeer was Simons- geboortenregister nog kinderen zandop zijn smalstnog altijd 300 De u'adgidsen Tjibbe Stelwagen en werden geboren, maar dat daarna meter breed.Simonszand is een flin- Menno de Leeuw zaten zaterdag- van de kaart verdween. Aan de hand ke plak zanddie ook bij hoogtij bo- avond beteuterd aan de koffie in de van oude zeekaaften en kerktorens ven het water uitsteekt.aan de kombuis van de Boschwad, het wist het genootschap te achterhalen Noordzeezijdezelfs met een strook schip dat hen naar Simonszand had waar Bosch destijds in zee verdronk lageduintjes. Tot voor kort dan. gebracht - een van de noordelijkste - de plaats waar nu Simonszand ligt. Want zaterdagkonden de weten- plekken van het land. Zaterdag was het extreem laag wa- schappersen gidsenmet eigenogen De geulen oostelijk en westelijk ter. Goed moment voor een expedi zien dat de duintjeshelemaal zijn van deze flinke zandplaat tussen tie. De Boschwad liet tegen drie uur verdwenen.Simonszand is nog Schiermonnikoog en Rottum zijn een gïoep wetenschappers en wad- slechtseen vlakke zandplaatdie bij dwars door de zandplaat gebroken. dengidsen van boord op het noorde- extreemhoogwater op eenfractie Dat heeft zulke enorme krachten lijke deel van Simonszand. Met na in zeeverdwijnt. losgemaakt dat deze plaat voor wad- schepjes, boren en zakjes gingen de En sporenvan het eilandBosch? lopers voortaan onbereikbaar is ge- onderzoekers het eiland te lijf.