Nomascus Hainanus )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Selection by Northern Yellow-Cheeked Crested Gibbons (Nomascus Annamensis)In Northern Cambodia
Food Selection by Northern Yellow-cheeked Crested Gibbons (Nomascus annamensis)in Northern Cambodia Naven Hon A thesis submitted to Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity School of Biological Sciences Victoria University of Wellington New Zealand 2016 i Abstract Tropical regions have extremely high plant diversity, which in turn supports a high diversity of animals. However, not all plant species are selected by animals as food sources, with some herbivores selecting only specific plants as food as not all plants have the same nutrient make up. Animals must select which food items to include in their diets, as the amount and type of nutrients in their diet can affect lifespan, health, fitness, and reproduction. Gibbon populations have declined significantly in recent years due to habitat destruction and hunting. Northern yellow-cheeked crested gibbon (Nomascus annamensis) is a newly described species, and has a limited distribution restricted to Cambodia, Laos and Vietnam. The northern yellow-cheeked crested gibbons play an important role in seed dispersal, yet little is currently known about this species, including its food selection and nutritional needs. However, data on food selection and nutritional composition of selected food items would greatly inform the conservation of both wild and captive populations of this species. This study aims to quantify food selection by the northern yellow-cheeked crested gibbons by investigating the main plant species consumed and the influence of the availability of food items on their selection. The study also explores the nutritional composition of food items consumed by this gibbon species and identifying key plant species that provide these significant nutrients. -

Gibbon Journal Nr
Gibbon Journal Nr. 5 – May 2009 Gibbon Conservation Alliance ii Gibbon Journal Nr. 5 – 2009 Impressum Gibbon Journal 5, May 2009 ISSN 1661-707X Publisher: Gibbon Conservation Alliance, Zürich, Switzerland http://www.gibbonconservation.org Editor: Thomas Geissmann, Anthropological Institute, University Zürich-Irchel, Universitätstrasse 190, CH–8057 Zürich, Switzerland. E-mail: [email protected] Editorial Assistants: Natasha Arora and Andrea von Allmen Cover legend Western hoolock gibbon (Hoolock hoolock), adult female, Yangon Zoo, Myanmar, 22 Nov. 2008. Photo: Thomas Geissmann. – Westlicher Hulock (Hoolock hoolock), erwachsenes Weibchen, Yangon Zoo, Myanmar, 22. Nov. 2008. Foto: Thomas Geissmann. ©2009 Gibbon Conservation Alliance, Switzerland, www.gibbonconservation.org Gibbon Journal Nr. 5 – 2009 iii GCA Contents / Inhalt Impressum......................................................................................................................................................................... i Instructions for authors................................................................................................................................................... iv Gabriella’s gibbon Simon M. Cutting .................................................................................................................................................1 Hoolock gibbon and biodiversity survey and training in southern Rakhine Yoma, Myanmar Thomas Geissmann, Mark Grindley, Frank Momberg, Ngwe Lwin, and Saw Moses .....................................4 -

10 Sota3 Chapter 7 REV11
200 Until recently, quantifying rates of tropical forest destruction was challenging and laborious. © Jabruson 2017 (www.jabruson.photoshelter.com) forest quantifying rates of tropical Until recently, Photo: State of the Apes Infrastructure Development and Ape Conservation 201 CHAPTER 7 Mapping Change in Ape Habitats: Forest Status, Loss, Protection and Future Risk Introduction This chapter examines the status of forested habitats used by apes, charismatic species that are almost exclusively forest-dependent. With one exception, the eastern hoolock, all ape species and their subspecies are classi- fied as endangered or critically endangered by the International Union for Conservation of Nature (IUCN) (IUCN, 2016c). Since apes require access to forested or wooded land- scapes, habitat loss represents a major cause of population decline, as does hunting in these settings (Geissmann, 2007; Hickey et al., 2013; Plumptre et al., 2016b; Stokes et al., 2010; Wich et al., 2008). Until recently, quantifying rates of trop- ical forest destruction was challenging and laborious, requiring advanced technical Chapter 7 Status of Apes 202 skills and the analysis of hundreds of satel- for all ape subspecies (Geissmann, 2007; lite images at a time (Gaveau, Wandono Tranquilli et al., 2012; Wich et al., 2008). and Setiabudi, 2007; LaPorte et al., 2007). In addition, the chapter projects future A new platform, Global Forest Watch habitat loss rates for each subspecies and (GFW), has revolutionized the use of satel- uses these results as one measure of threat lite imagery, enabling the first in-depth to their long-term survival. GFW’s new analysis of changes in forest availability in online forest monitoring and alert system, the ranges of 22 great ape and gibbon spe- entitled Global Land Analysis and Dis- cies, totaling 38 subspecies (GFW, 2014; covery (GLAD) alerts, combines cutting- Hansen et al., 2013; IUCN, 2016c; Max Planck edge algorithms, satellite technology and Insti tute, n.d.-b). -

An Efficient Method for Simulating Typhoon Waves Based on A
Journal of Marine Science and Engineering Article An Efficient Method for Simulating Typhoon Waves Based on a Modified Holland Vortex Model Lvqing Wang 1,2,3, Zhaozi Zhang 1,*, Bingchen Liang 1,2,*, Dongyoung Lee 4 and Shaoyang Luo 3 1 Shandong Province Key Laboratory of Ocean Engineering, Ocean University of China, 238 Songling Road, Qingdao 266100, China; [email protected] 2 College of Engineering, Ocean University of China, 238 Songling Road, Qingdao 266100, China 3 NAVAL Research Academy, Beijing 100070, China; [email protected] 4 Korea Institute of Ocean, Science and Technology, Busan 600-011, Korea; [email protected] * Correspondence: [email protected] (Z.Z.); [email protected] (B.L.) Received: 20 January 2020; Accepted: 23 February 2020; Published: 6 March 2020 Abstract: A combination of the WAVEWATCH III (WW3) model and a modified Holland vortex model is developed and studied in the present work. The Holland 2010 model is modified with two improvements: the first is a new scaling parameter, bs, that is formulated with information about the maximum wind speed (vms) and the typhoon’s forward movement velocity (vt); the second is the introduction of an asymmetric typhoon structure. In order to convert the wind speed, as reconstructed by the modified Holland model, from 1-min averaged wind inputs into 10-min averaged wind inputs to force the WW3 model, a gust factor (gf) is fitted in accordance with practical test cases. Validation against wave buoy data proves that the combination of the two models through the gust factor is robust for the estimation of typhoon waves. -

Sound Spectrum Characteristics of Eastern Black Crested Gibbons
NORTH-WESTERN JOURNAL OF ZOOLOGY 13 (2): 347-351 ©NwjZ, Oradea, Romania, 2017 Article No.: e161705 http://biozoojournals.ro/nwjz/index.html Sound spectrum characteristics of Eastern Black Crested Gibbons Huaiqing DENG#, Huamei WEN# and Jiang ZHOU* School of Life Sciences, Guizhou Normal University, Guiyang, Guizhou, 550001, China, E-mail: [email protected] # These authors contributed to the work equally and regarded as Co-first authors. * Corresponding author, J. Zhou, E-mail: [email protected], Tel.:13985463226 Received: 01. November 2016 / Accepted: 07. September 2016 / Available online: 19. September 2016 / Printed: December 2017 Abstract. Studies about the sound spectrum characteristics and the intergroup differences in eastern black crested gibbon (Nomascus nasutus) song are still rare. Here, we studied the singing behavior of eastern black crested gibbon based on song samples of three groups of gibbons collected from Trung Khanh, northern Vietnam. The results show that: 1) Song frequency of both adult male and adult female eastern black crested gibbon is low and both are below 2 KHz; 2) Songs of adult male eastern black gibbons are composed mainly of short syllables (aa notes) and frequency modulated syllables (FM notes), while adult female gibbons only produce a stable and stereotyped pattern of great calls; 3) There is significant differences among the three groups in highest and lowest frequency of FM syllable in males’ song; 4) The song chorus is dominated by adult males, while females add a great call; 5) The sound spectrum frequency is lower and complex, which is different from Hainan gibbon. The low frequency in the singing of the eastern black crested gibbon is related to the structure and low quality of the vegetation of its habitat. -
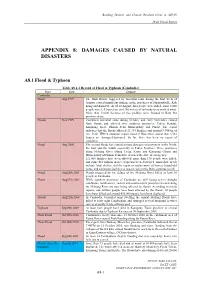
Appendix 8: Damages Caused by Natural Disasters
Building Disaster and Climate Resilient Cities in ASEAN Draft Finnal Report APPENDIX 8: DAMAGES CAUSED BY NATURAL DISASTERS A8.1 Flood & Typhoon Table A8.1.1 Record of Flood & Typhoon (Cambodia) Place Date Damage Cambodia Flood Aug 1999 The flash floods, triggered by torrential rains during the first week of August, caused significant damage in the provinces of Sihanoukville, Koh Kong and Kam Pot. As of 10 August, four people were killed, some 8,000 people were left homeless, and 200 meters of railroads were washed away. More than 12,000 hectares of rice paddies were flooded in Kam Pot province alone. Floods Nov 1999 Continued torrential rains during October and early November caused flash floods and affected five southern provinces: Takeo, Kandal, Kampong Speu, Phnom Penh Municipality and Pursat. The report indicates that the floods affected 21,334 families and around 9,900 ha of rice field. IFRC's situation report dated 9 November stated that 3,561 houses are damaged/destroyed. So far, there has been no report of casualties. Flood Aug 2000 The second floods has caused serious damages on provinces in the North, the East and the South, especially in Takeo Province. Three provinces along Mekong River (Stung Treng, Kratie and Kompong Cham) and Municipality of Phnom Penh have declared the state of emergency. 121,000 families have been affected, more than 170 people were killed, and some $10 million in rice crops has been destroyed. Immediate needs include food, shelter, and the repair or replacement of homes, household items, and sanitation facilities as water levels in the Delta continue to fall. -

Visitor Attraction Trends England 2003 Presents the Findings of the Survey of Visits to Visitor Attractions Undertaken in England by Visitbritain
Visitor Attraction Trends England 2003 ACKNOWLEDGEMENTS VisitBritain would like to thank all representatives and operators in the attraction sector who provided information for the national survey on which this report is based. No part of this publication may be reproduced for commercial purp oses without previous written consent of VisitBritain. Extracts may be quoted if the source is acknowledged. Statistics in this report are given in good faith on the basis of information provided by proprietors of attractions. VisitBritain regrets it can not guarantee the accuracy of the information contained in this report nor accept responsibility for error or misrepresentation. Published by VisitBritain (incorporated under the 1969 Development of Tourism Act as the British Tourist Authority) © 2004 Bri tish Tourist Authority (trading as VisitBritain) Cover images © www.britainonview.com From left to right: Alnwick Castle, Legoland Windsor, Kent and East Sussex Railway, Royal Academy of Arts, Penshurst Place VisitBritain is grateful to English Heritage and the MLA for their financial support for the 2003 survey. ISBN 0 7095 8022 3 September 2004 VISITOR ATTR ACTION TRENDS ENGLAND 2003 2 CONTENTS CONTENTS A KEY FINDINGS 4 1 INTRODUCTION AND BACKGROUND 12 1.1 Research objectives 12 1.2 Survey method 13 1.3 Population, sample and response rate 13 1.4 Guide to the tables 15 2 ENGLAND VISIT TRENDS 2002 -2003 17 2.1 England visit trends 2002 -2003 by attraction category 17 2.2 England visit trends 2002 -2003 by admission type 18 2.3 England visit trends -

An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan
CENTER FOR EXCELLENCE IN DISASTER MANAGEMENT & HUMANITARIAN ASSISTANCE An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan WWW.CFE-DMHA.ORG Contents Introduction ...........................................................................................................................2 Humanitarian Assistance and Disaster Relief (HADR) Organizations ..................................3 Search and Rescue (SAR) Organizations ..........................................................................18 Appendix A: Taiwan Foreign Disaster Relief Assistance ....................................................29 Appendix B: DOD/USINDOPACOM Disaster Relief in Taiwan ...........................................31 Appendix C: Taiwan Central Government Disaster Management Structure .......................34 An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan 1 Introduction This information paper serves as an introduction to the major Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) organizations in Taiwan and international organizations working with Taiwanese government organizations or non-governmental organizations (NGOs) in HADR. The paper is divided into two parts: The first section focuses on major International Non-Governmental Organizations (INGOs), and local NGO partners, as well as international Civil Society Organizations (CSOs) working in HADR in Taiwan or having provided -

EAZA NEWS Zoo Nutrition 4
ZOO NUTRITION EAZANEWS 2008 publication of the european association of zoos and aquaria september 2008 — eaza news zoo nutrition issue number 4 8 Feeding our animals without wasting our planet 10 Sustainability and nutrition of The Deep’s animal feed sources 18 Setting up a nutrition research programme at Twycross Zoo 21 Should zoo food be chopped? 26 Feeding practices for captive okapi 15 The development of a dietary review team 24 Feeding live prey; chasing away visitors? EAZA Zoonutr5|12.indd 1 08-09-2008 13:50:55 eaza news 2008 colophon zoo nutrition EAZA News is the quarterly magazine of the European Association of Zoos and Aquaria (EAZA) issue 4 Managing Editor Jeannette van Benthem ([email protected]) Editorial staff for EAZA News Zoo Nutrition Issue 4 Joeke Nijboer, Andrea Fidgett, Catherine King Design Jantijn Ontwerp bno, Made, the Netherlands Printing Drukkerij Van den Dool, Sliedrecht, the Netherlands ISSN 1574-2997. The views expressed in this newsletter are not necessarily those of the European Association of Zoos and Aquaria. Printed on TREE-FREE paper bleached without chlorine and free from acid who is who in eaza foreword EAZA Executive Committee Although nourishing zoo animals properly and according chair Leobert de Boer, Apenheul Primate Park vice-chair Simon Tonge, Paignton Zoo secretary Eric Bairrao Ruivo, Lisbon Zoo treasurer Ryszard Topola, Lodz Zoo to their species’ needs is a most basic requirement to chair eep committee Bengt Holst, Copenhagen Zoo chair membership & ethics maintain sustainable populations in captivity, zoo and committee Lars Lunding Andersen, Copenhagen Zoo chair aquarium committee aquarium nutrition has been a somewhat underestimated chair legislation committee Jurgen Lange, Berlin Zoo Ulrich Schurer, Wuppertal Zoo science for a long time. -

In Our Hands: the British and UKOT Species That Large Charitable Zoos & Aquariums Are Holding Back from Extinction (AICHI Target 12)
In our hands: The British and UKOT species that Large Charitable Zoos & Aquariums are holding back from extinction (AICHI target 12) We are: Clifton & West of England Zoological Society (Bristol Zoo, Wild Places) est. 1835 Durrell Wildlife Conservation Trust (Jersey Zoo) est. 1963 East Midland Zoological Society (Twycross Zoo) est. 1963 Marwell Wildlife (Marwell Zoo) est. 1972 North of England Zoological Society (Chester Zoo) est. 1931 Royal Zoological Society of Scotland (Edinburgh Zoo, Highland Wildlife Park) est. 1913 The Deep est. 2002 Wild Planet Trust (Paignton Zoo, Living Coasts, Newquay Zoo) est. 1923 Zoological Society of London (ZSL London Zoo, ZSL Whipsnade Zoo) est. 1826 1. Wildcat 2. Great sundew 3. Mountain chicken 4. Red-billed chough 5. Large heath butterfly 6. Bermuda skink 7. Corncrake 8. Strapwort 9. Sand lizard 10. Llangollen whitebeam 11. White-clawed crayfish 12. Agile frog 13. Field cricket 14. Greater Bermuda snail 15. Pine hoverfly 16. Hazel dormouse 17. Maiden pink 18. Chagos brain coral 19. European eel 2 Executive Summary: There are at least 76 species native to the UK, Crown Dependencies, and British Overseas Territories which Large Charitable Zoos & Aquariums are restoring. Of these: There are 20 animal species in the UK & Crown Dependencies which would face significant declines or extinction on a global, national, or local scale without the action of our Zoos. There are a further 9 animal species in the British Overseas Territories which would face significant declines or extinction without the action of our Zoos. These species are all listed as threatened on the IUCN Red List. There are at least 19 UK animal species where the expertise of our Zoological Institutions is being used to assist with species recovery. -

A White-Cheeked Crested Gibbon Ethogram & a Comparison Between Siamang
A white-cheeked crested gibbon ethogram & A comparison between siamang (Symphalangus syndactylus) and white-cheeked crested gibbon (Nomascus leucogenys) Janet de Vries Juli – November 2004 The gibbon research Lab., Zürich (Zwitserland) Van Hall Instituut, Leeuwarden J. de Vries: Ethogram of the White-Cheeked Crested Gibbon 2 A white-cheeked crested gibbon ethogram A comparison between siamang (Symphalangus syndactylus) and white-cheeked crested gibbon (Nomascus leucogenys) By: Janet de Vries Final project Animal management Projectnumber: 344311 Juli 2004 – November 2004-12-01 Van Hall Institute Supervisor: Thomas Geissmann of the Gibbon Research Lab Supervisors: Marcella Dobbelaar, & Celine Verheijen of Van Hall Institute Keywords: White-cheeked crested gibbon (Nomascus leucogenys), Siamang (Symphalangus syndactylus), ethogram, behaviour elements. J. de Vries: Ethogram of the White-Cheeked Crested Gibbon 3 Preface This project… text missing Janet de Vries Leeuwarden, November 2004 J. de Vries: Ethogram of the White-Cheeked Crested Gibbon 4 Contents Summary ................................................................................................................................ 5 1. Introduction ........................................................................................................................ 6 1.1 Gibbon Ethograms ..................................................................................................... 6 1.2 Goal .......................................................................................................................... -

University of Stirling Clare Cunningham Department Of
UNIVERSITY OF STIRLING CLARE CUNNINGHAM DEPARTMENT OF PSYCHOLOGY Cognitive Flexibility in Gibbons (Hylobatidae): Object Manipulation and Tool-Use Submitted for the degree of Doctor of Philosophy Supervisor Dr James Anderson September 2006 1 Acknowledgements First and foremost, my thanks go to James Anderson, my supervisor during this research. I thank him for his help and support during the last three years and his considerable help in revising and editing. Also, to Hannah Buchanan-Smith whose comments and help have been invaluable. To Alan Mootnick at the Gibbon Conservation Center (GCC), California, USA, I offer my sincerest gratitude for allowing access to the gibbons and his home during data collection. I also thank Erin Bell, facility supervisor at GCC, who was most supportive during my time there. The staff and management at Twycross Zoo, West Midlands, UK, were also very helpful and accommodating. To my family and partner, I offer my thanks; although there have been times when I have been difficult to live with during the past few years, their support has been unwavering. My particular thanks go to Mark Miller for listening to me talk about gibbons and for proof reading some of this thesis. I would also like to thank the Evolutionary Psychology group at the University of Liverpool, Robin Dunbar, John Lycett, Susanne Shultz and Craig Roberts. They have been very understanding while I have been completing this thesis and willing to offer help and discussion at all times. I am also thankful to my examiners for their time in reading this work. Finally, to the gibbons who made the many hours of ape-watching enjoyable, informative and frustrating.