DESIDERATA LIST, January, 2014 MAMMAL IMAGES LIBRARY AMERICAN SOCIETY of MAMMALOGISTS the Following Taxa Are Not Represented In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of the Mammals of Indonesia
CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation i ii CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation By Ibnu Maryanto Maharadatunkamsi Anang Setiawan Achmadi Sigit Wiantoro Eko Sulistyadi Masaaki Yoneda Agustinus Suyanto Jito Sugardjito RESEARCH CENTER FOR BIOLOGY INDONESIAN INSTITUTE OF SCIENCES (LIPI) iii © 2019 RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI) Cataloging in Publication Data. CHECKLIST OF THE MAMMALS OF INDONESIA: Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation/ Ibnu Maryanto, Maharadatunkamsi, Anang Setiawan Achmadi, Sigit Wiantoro, Eko Sulistyadi, Masaaki Yoneda, Agustinus Suyanto, & Jito Sugardjito. ix+ 66 pp; 21 x 29,7 cm ISBN: 978-979-579-108-9 1. Checklist of mammals 2. Indonesia Cover Desain : Eko Harsono Photo : I. Maryanto Third Edition : December 2019 Published by: RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI). Jl Raya Jakarta-Bogor, Km 46, Cibinong, Bogor, Jawa Barat 16911 Telp: 021-87907604/87907636; Fax: 021-87907612 Email: [email protected] . iv PREFACE TO THIRD EDITION This book is a third edition of checklist of the Mammals of Indonesia. The new edition provides remarkable information in several ways compare to the first and second editions, the remarks column contain the abbreviation of the specific island distributions, synonym and specific location. Thus, in this edition we are also corrected the distribution of some species including some new additional species in accordance with the discovery of new species in Indonesia. -

A Species-Level Phylogenetic Supertree of Marsupials
J. Zool., Lond. (2004) 264, 11–31 C 2004 The Zoological Society of London Printed in the United Kingdom DOI:10.1017/S0952836904005539 A species-level phylogenetic supertree of marsupials Marcel Cardillo1,2*, Olaf R. P. Bininda-Emonds3, Elizabeth Boakes1,2 and Andy Purvis1 1 Department of Biological Sciences, Imperial College London, Silwood Park, Ascot SL5 7PY, U.K. 2 Institute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, U.K. 3 Lehrstuhl fur¨ Tierzucht, Technical University of Munich, Alte Akademie 12, 85354 Freising-Weihenstephan, Germany (Accepted 26 January 2004) Abstract Comparative studies require information on phylogenetic relationships, but complete species-level phylogenetic trees of large clades are difficult to produce. One solution is to combine algorithmically many small trees into a single, larger supertree. Here we present a virtually complete, species-level phylogeny of the marsupials (Mammalia: Metatheria), built by combining 158 phylogenetic estimates published since 1980, using matrix representation with parsimony. The supertree is well resolved overall (73.7%), although resolution varies across the tree, indicating variation both in the amount of phylogenetic information available for different taxa, and the degree of conflict among phylogenetic estimates. In particular, the supertree shows poor resolution within the American marsupial taxa, reflecting a relative lack of systematic effort compared to the Australasian taxa. There are also important differences in supertrees based on source phylogenies published before 1995 and those published more recently. The supertree can be viewed as a meta-analysis of marsupial phylogenetic studies, and should be useful as a framework for phylogenetically explicit comparative studies of marsupial evolution and ecology. -
Supporting Information
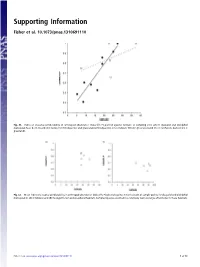
Supporting Information Fisher et al. 10.1073/pnas.1310691110 Fig. S1. Index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against latitude of sampling sites where dasyurid and didelphid marsupials have been recorded in rainforest (filled points) and grassland (unfilled points). Lines indicate fitted regressions (solid line = rainforest, dashed line = grassland). Fig. S2. Mean index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against mean latitude of sample points for dasyurid and didelphid marsupials in (A) shrubland and (B) Eucalypt forest and woodland habitats. Sampled species occurred in a relatively narrow range of latitudes in these habitats. Fisher et al. www.pnas.org/cgi/content/short/1310691110 1of13 Fig. S3. Phylogeny of insectivorous marsupials with known life history data, based on ref. 1 with updates from ref. 2. 1. Cardillo M, Bininda-Emonds ORP, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264(1):11–31. 2. Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol Lett 12(6):538–549. Fisher et al. www.pnas.org/cgi/content/short/1310691110 2of13 Fisher et al. Table S1. Reproductive traits and diets of insectivorous marsupials Latitude (south) www.pnas.org/cgi/content/short/1310691110 Proportion Proportion Female for Habitat of of age species class for females males Male Breeding Copulation Litters Scrotal at first with species Genus species -

The Mammals of Southern West Sepik Province, Papua New Guinea: Their Distribution, Abundance, Human Use and Zoogeography
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Flannery, Tim F., & Seri, L., 1990. The mammals of southern West Sepik Province, Papua New Guinea: their distribution, abundance, human use and zoogeography. Records of the Australian Museum 42(2): 173–208. [6 July 1990]. doi:10.3853/j.0067-1975.42.1990.114 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia Records of the Australian Museum (1990) Vol. 42: 173-208. ISSN 0067 1975 173 The Mammals of Southern West Sepik Province, Papua New Guinea: their Distribution, Abundance, Human Use and Zoogeography T.F. FLANNERyl & L. SERI2 IThe Australian Museum, 6 College Street, Sydney, NSW 2000, Australia 2Department of Environment and Conservation, Division of Wildlife, P.O. Box 6601, Boroko, Papua New Guinea ABSTRACT. A mammal survey was carried out between 1984 and 1987 in southern West Sepik Province, Papua New Guinea. Eleven major collecting localities, as well as some more minor ones, lying at altitudes of between 120 and 3,200 m were investigated. Voucher specimens for 87 indigenous mammal taxa were obtained, but research suggests that mammal diversity in the area may be as high as 120 species. This is the highest mammal diversity recorded anywhere in Australasia. A similar high bird diversity suggests that the area may be one of exceptionally high biodiversity overall. The most diverse mammal assemblages in the study area are found in the midmontane oak forests (between 1,500 and 2,500 m). -

2014 Annual Reports of the Trustees, Standing Committees, Affiliates, and Ombudspersons
American Society of Mammalogists Annual Reports of the Trustees, Standing Committees, Affiliates, and Ombudspersons 94th Annual Meeting Renaissance Convention Center Hotel Oklahoma City, Oklahoma 6-10 June 2014 1 Table of Contents I. Secretary-Treasurers Report ....................................................................................................... 3 II. ASM Board of Trustees ............................................................................................................ 10 III. Standing Committees .............................................................................................................. 12 Animal Care and Use Committee .......................................................................... 12 Archives Committee ............................................................................................... 14 Checklist Committee .............................................................................................. 15 Conservation Committee ....................................................................................... 17 Conservation Awards Committee .......................................................................... 18 Coordination Committee ....................................................................................... 19 Development Committee ........................................................................................ 20 Education and Graduate Students Committee ....................................................... 22 Grants-in-Aid Committee -

Natural History of the Metatheria
FAUNA of AUSTRALIA 18. NATURAL HISTORY OF THE METATHERIA ELEANOR M. RUSSELL, A.K. LEE & GEORGE R. WILSON 1 18. NATURAL HISTORY OF THE METATHERIA 2 18. NATURAL HISTORY OF THE METATHERIA NATURAL HISTORY Ecology Diet. Traditionally, Australian marsupials have been viewed in two dietary categories: the predominantly carnivorous marsupials, with polyprotodont dentition, and the predominantly herbivorous large diprotodonts. Recent observations on feeding (Smith 1982a; Henry & Craig 1984) and analysis of diet (Hall 1980a; Statham 1982) have not altered this view in the broadest sense. They have shown, however, that some polyprotodonts such as the peramelids (bandicoots) (Heinsohn 1966; Lee & Cockburn 1985) and the Bilby, Macrotis lagotis (Johnson 1980a), include significant proportions of plant items in their diets. Further, some small and medium-sized diprotodonts are now known to be dependent upon plant products such as nectar, pollen, plant exudates, fruit and seed (Smith 1982a; Mansergh 1984b; Turner 1984a, 1984b; Lee & Cockburn 1985). Arthropods appear to be the principal food items of most dasyurids (for example, Antechinus species, quolls, phascogales and planigales) (Morton 1978d; Blackall 1980; Hall 1980a; Statham 1982; Lee & Cockburn 1985), peramelids (Heinsohn 1966; Lee & Cockburn 1985), the Bilby (Johnson 1980a), the Marsupial Mole, Notoryctes typhlops (Corbett 1975), the Numbat, Myrmecobius fasciatus (Calaby 1960) and the Striped Possum, Dactylopsila trivirgata (Smith 1982b). Arthropods also are consistent, but not predominant, food items of burramyids (pygmy-possums) (Turner 1984a, 1984b), petaurids (ring-tail possums and gliders) (Smith 1982a, 1984a; Smith & Russell 1982) and occasionally occur in the diet of the Common Brushtail Possum, Trichosurus vulpecula (Kerle 1984c). Vertebrates appear to be important in the diet of some large dasyurids, although many feed predominantly upon insects (Blackall 1980; Godsell 1982). -

Thomas Mutton Thesis
EVOLUTIONARY BIOLOGY OF THE AUSTRALIAN CARNIVOROUS MARSUPIAL GENUS ANTECHINUS Thomas Yeshe Mutton B. App. Sci. (Hons) School of Earth, Environmental and Biological Sciences Science and Engineering Faculty Queensland University of Technology Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy i Keywords Antechinus, Australia, Australian mesic zone, biogeographical barriers, biogeography, breeding biology, congeneric competition, conservation, genetic structure, Dasyuridae, dasyurid, life-history, mammal, mammalian, Miocene, molecular systematics, Phascogalini, phylogenetics, phylogeography, Pleistocene, Pliocene, population genetics, Queensland, systematics (Davison ) ii Abstract Antechinus is an Australian genus of small carnivorous marsupials. Since , the number of described species in the genus has increased by % from ten to fifteen. The systematic relationships of these new species and others in the genus have not been well resolved and a broad phylogeographic study of the genus is lacking. Moreover, little ecological information is known about these new species. Therefore, the present study examined the evolutionary biology of Antechinus in two complimentary components. The first component aimed to resolve the systematics and phylogeography of the genus Antechinus. The second component, at a finer spatiotemporal scale, aimed to improve understanding of the autecology, habitat use and risk of extinction within the group, with a focus on the recently named buff-footed antechinus, A. mysticus and a partially sympatric congener, A. subtropicus. To date, powerful (>two gene) molecular studies have only included eight Antechinus species. Here, the first comprehensive, multi-gene phylogenetic analysis of the genus using six genes was provided, incorporating all known species and subspecies of Antechinus. Four main lineages of Antechinus were reconstructed: () The dusky antechinus (A. -

Chapter 8 Biodiversity and Biogeography of the Moss-Mice of New Guinea: a Taxonomic Revision of Pseudohydromys (Muridae: Murinae
Chapter 8 Biodiversity and Biogeography of the Moss-Mice of New Guinea: A Taxonomic Revision of Pseudohydromys (Muridae: Murinae) KRISTOFER M. HELGEN1 AND LAUREN E. HELGEN2 ABSTRACT Morphological investigations involving nearly all available museum material representing New Guinea ‘‘moss-mice’’ (rodents traditionally classified in the genera Pseudohydromys, Neohydromys, Mayermys,andMicrohydromys) reveal outstanding undiagnosed taxic diversity (a minimum of 16 species, versus the eight species previously described) and allow for redefinition of generic boundaries among these little-studied rodents. Apart from Micro- hydromys Tate and Archbold, 1941 (comprising two species, as recently revised by Helgen et al., in press), herein we recognize two genera of New Guinea moss-mice: Pseudohydromys Ru¨mmler, 1934 (now incorporating Neohydromys Laurie, 1952, Mayermys Laurie and Hill, 1954, and ‘‘Microhydromys’’ musseri Flannery, 1989) and a newly described genus, Mirzamys. Species of Pseudohydromys are recorded from montane areas throughout New Guinea (elevations spanning 600 to at least 3800 meters), including the mountain ranges of the Central Cordillera, the Huon Peninsula, and the North Coastal ranges. We diagnose and review 12 species of Pseudohydromys, including six species described as new. The new genus Mirzamys is erected to accommodate two newly described species of small terrestrial rodents from middle and upper montane forests and subalpine grassland edges (1900–3450 m) in the mountains of central New Guinea. Together these two new species represent a distinctive hydromyin lineage that resembles the terrestrial New Guinea hydromyin genera Pseudohydromys and Paraleptomys in various traits. Ecological attributes of all recognized moss-mice taxa, both previously and newly described, are reviewed in light of all information currently available about their biology. -

Vertebrate Pests Committee List of Exotic Vertebrate Animals in Australia, May 2003……………………………………………………
Vertebrate Pests Committee List of Exotic Vertebrate Animals in Australia May 2003 Contact Details Background Vertebrate Pests Committee Secretary All species on this list (excluding those with B or C c\o Land Protection annotations) form a definitive record of the non- Department of Natural Resources & Mines indigenous vertebrate mammals, birds, amphibians GPO Box 2454 and reptiles held in Australia under State and Territory BRISBANE QLD 4001 legislation. (It is the responsibility of the holder of an Tel. 07 3405 5540 individual animal to ensure that they are also Fax. 07 3405 5551 compliant with Commonwealth legislation relating to Mobile. the possession and quarantine of exotic animals.) Email. [email protected] This list should be used as a reference by the Vertebrate Pests Committee and Commonwealth, Sustainable Wildlife Industries State and Territory agencies in controlling the entry, Environment Australia movement and keeping of exotic vertebrate animals. GPO Box 787 CANBERRA ACT 2601 This list may be subject to change from time to time Tel. 02 6274 2880 (with VPC approval), to incorporate changes in the Fax. 02 6274 1921Email. [email protected] taxonomic name of species. It may also be changed where additions have been made through the legal importation of new exotic species into Australia under References the provisions of the Environmental Protection and Biodiversity Conservation Act 1999 and the Christidis, L. and Boles, W. E. 1994. The Taxonomy Quarantine Act 1908. and Species of Birds of Australia and its Territories. RAOU Monograph 2. Each species, unless otherwise stated, has so far only be subjected to a general assessment of the risk it Frost, N.C. -
Phascogale Calura)
Reproductive strategies of the red-tailed phascogale (Phascogale calura) Wendy Foster B.Sc. (Hons) Department of Ecology and Evolutionary Biology School of Earth and Environmental Sciences The University of Adelaide South Australia March 2008 A thesis submitted for the degree of Doctor of Philosophy at The University of Adelaide Who’s Your Daddy? Over the last year, there has been some action happening quietly behind the doors of the Animal Health and Research Centre, and I’m not referring to the treatment of sick animals. I’m talking about sex, and lots of it! This is Meanwhile, mum is left with eight babies to the six-hour continuous lovemaking that has raise without a single child support you hanging from the roof kind of sex. The payment. Luckily the young are born new partner every day kind of sex. The make smaller than a tic-tac, which makes giving love til you drop kind of sex. birth easier. Of the fourteen or so young born, only eight manage to find a teat – for What! I hear you exclaim. That’s outrageous! the others it is just bad luck. Mum carts the kids around continuously for about seven Well before you get too outraged maybe I weeks, then decides they are big enough to should clarify a couple of things. The stay in the nest while she goes ‘out on the individuals involved in this rampant love- tree’. Another seven weeks later and the making are an endangered carnivorous kids are finally ready to leave home, having marsupial known as the red-tailed phascogale. -
Author Index
Author Index Abbie, A. A., 10, 23, 240, 243-6, 248, Banks, M. R., 67, 94 250-1, 253, 257 Barber, H. N., 34, 37,47 Adams, J., 444, 449 Barbour, R. A., 235, 244, 250- l, 257-8 Adams, N. M., 169, 178 von Bardeleben, K., 426, 449 Adams, W. E., 252, 257-8 Bardin, C. W., 440, 449 Adey, W. R., 248-9, 258 Barker, I., 214-15, 218 Adie, R. J ., 80- l, 88 Barker, I. K., 139-41, 144, 149, 151 Agar, W. E., 426, 449 Barker, P. F., 78, 88 Ahmad, K., 39,4 7 Barker, R., 221, 232 Air, G. M., 27, 45, 100, Ill Barker, S., 141, 144, 146-7, 151 Aitken, P. F., 15, 20, 23 Barnett, C. H., 242,258,416,419,449 Alexandre, H. L., 275, 283 Barnett,J.L., 149,151,211-12,219,347, Allard, G. 0., 75, 88 349,354-5,357,370,373 Allen, J. A., 14 Barr, K. W., 74, 88 Allen, J. M., 242, 265,446,453 Barsbold, R., 71, 88 Allison, A., 193, 208 Bartholomew, G. A., 229, 232 Amoroso, E. C., 240, 258, 340, 343 Barton, A. A., 254, 262 Anderson, D., 364, 367, 373, 398, 399, 408 Barton, C. M., 81, 88 Anderson, J. J., 103, Ill Basmaijan, J. V., 293, 304 Anderson, N., 143, 151 Bautista, N. S., 248, 258 Anderson, T. A., 74, 88 de Bavay, J. M., 238-40,260,268 Andrew, W., 255, 258 Baxter, J. S., 239-40, 258 Appelbaum, E., 243, 267 Beck, R. R., 352,374,400-1,409 Archer, M., 12, 15, 19-20, 23-4, 316-19, Beddard,F.E., 411,449 422 Beer, A. -

Total Evidence Phylogeny and Evolutionary Timescale for Australian Faunivorous Marsupials (Dasyuromorphia) Shimona Kealy1 and Robin Beck2*
Kealy and Beck BMC Evolutionary Biology (2017) 17:240 DOI 10.1186/s12862-017-1090-0 RESEARCHARTICLE Open Access Total evidence phylogeny and evolutionary timescale for Australian faunivorous marsupials (Dasyuromorphia) Shimona Kealy1 and Robin Beck2* Abstract Background: The order Dasyuromorphia is a diverse radiation of faunivorous marsupials, comprising >80 modern species in Australia and New Guinea. It includes dasyurids, the numbat (the myrmecobiid Myrmecobius fasciatus) and the recently extinct thylacine (the thylacinid Thylacinus cyncocephalus). There is also a diverse fossil record of dasyuromorphians and “dasyuromorphian-like” taxa known from Australia. We present the first total evidence phylogenetic analyses of the order, based on combined morphological and molecular data (including a novel set of 115 postcranial characters), to resolve relationships and calculate divergence dates. We use this information to analyse the diversification dynamics of modern dasyuromorphians. Results: Our morphology-only analyses are poorly resolved, but our molecular and total evidence analyses confidently resolve most relationships within the order, and are strongly congruent with recent molecular studies. Thylacinidae is the first family to diverge within the order, and there is strong support for four tribes within Dasyuridae (Dasyurini, Phascogalini, Planigalini and Sminthopsini). Among fossil taxa, Ankotarinja and Keeuna do not appear to be members of Dasyuromorphia, whilst Barinya and Mutpuracinus are of uncertain relationships within the