Prescribing Newsletter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Potentially Inappropriate Prescriptions (Pips) for Older People (Modified from ‘STOPP/START 2’ O’Mahony Et Al 2014)
Appendix A: Potentially Inappropriate Prescriptions (PIPs) for older people (modified from ‘STOPP/START 2’ O’Mahony et al 2014) Consider holding (or deprescribing - consult with patient): 1. Any drug prescribed without an evidence-based clinical indication 2. Any drug prescribed beyond the recommended duration, where well-defined 3. Any duplicate drug class (optimise monotherapy) Avoid hazardous combinations e.g.: 1. The Triple Whammy: NSAID + ACE/ARB + diuretic in all ≥ 65 year olds (NHS Scotland 2015) 2. Sick Day Rules drugs: Metformin or ACEi/ARB or a diuretic or NSAID in ≥ 65 year olds presenting with dehydration and/or acute kidney injury (AKI) (NHS Scotland 2015) 3. Anticholinergic Burden (ACB): Any additional medicine with anticholinergic properties when already on an Anticholinergic/antimuscarinic (listed overleaf) in > 65 year olds (risk of falls, increased anticholinergic toxicity: confusion, agitation, acute glaucoma, urinary retention, constipation). The following are known to contribute to the ACB: Amantadine Antidepressants, tricyclic: Amitriptyline, Clomipramine, Dosulepin, Doxepin, Imipramine, Nortriptyline, Trimipramine and SSRIs: Fluoxetine, Paroxetine Antihistamines, first generation (sedating): Clemastine, Chlorphenamine, Cyproheptadine, Diphenhydramine/-hydrinate, Hydroxyzine, Promethazine; also Cetirizine, Loratidine Antipsychotics: especially Clozapine, Fluphenazine, Haloperidol, Olanzepine, and phenothiazines e.g. Prochlorperazine, Trifluoperazine Baclofen Carbamazepine Disopyramide Loperamide Oxcarbazepine Pethidine -

Chlorphenamine Maleate)
Package leaflet: Information for the patient Chlorphenamine 10 mg/ml Solution for Injection (Chlorphenamine Maleate) Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. − Keep this leaflet. You may need to read it again. − If you have any further questions, ask your doctor or nurse. − If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Chlorphenamine is and what it is used for 2. What you need to know before Chlorphenamine is given 3. How Chlorphenamine is given 4. Possible side effects 5. How to store Chlorphenamine 6. Contents of the pack and other information 1. What Chlorphenamine is and what it is used for Chlorphenamine 10 mg/ml Solution for Injection contains the active ingredient chlorphenamine maleate which is an antihistamine. Chlorphenamine is indicated in adults and children (aged 1 month to 18 years) for the treatment of acute allergic reactions. These medicines inhibit the release of histamine into the body that occurs during an allergic reaction. This product relieves some of the main symptoms of a severe allergic reaction. 2. What you need to know before Chlorphenamine is given You MUST NOT be given Chlorphenamine: if you are allergic to chlorphenamine maleate or any of the other ingredients of this medicine (listed in section 6) if you have had monoamine oxidase inhibitor (MAOI) antidepressive treatment within the past 14 days. Warnings and precautions Talk to your doctor or nurse before you are given this medicine if you: are being treated for an overactive thyroid or enlarged prostate gland have epilepsy, raised blood pressure within the eye or glaucoma, very high blood pressure, heart, liver, asthma or other chest diseases. -

Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj
Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj Nehad J. Ahmed1*, Menshawy A. Menshawy2 1Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia, 2Department of Medicinal chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia Abstract Aim: This study aims to illustrate the prescribing trends of antihistamines in the outpatient setting in Al-Kharj. Materials and Methods: This is a retrospective study that included the evaluation of antihistamines in the outpatient setting in a public hospital in Al-Kharj. The data were collected from the pharmacy-based computer system. Results: The total number of prescriptions that included antihistamines was 799. Most of the prescribed ORIGINAL ARTICLE ARTICLE ORIGINAL antihistamines were first-generation sedating antihistamines (chlorphenamine and diphenhydramine) (66.33%). About 63.20% of the prescribed antihistamines included chlorpheniramine followed by cetirizine (19.27%) and loratadine (14.39%). Conclusion: Antihistamines were prescribed commonly in the outpatient setting mainly first-generation sedating antihistamines. It is recommended to increase the awareness of health- care providers about the efficacy and the side effects of antihistamines and to encourage them to use these agents wisely. Key words: Antihistamines, outpatient, prescribing, trends INTRODUCTION In addition, antihistamines have been classified as sedating antihistamines (first-generation antihistamines) and non- ntihistamines are used in the sedating antihistamines (second-generation antihistamines).[4] management of allergic conditions. Sedating antihistamines include chlorphenamine, clemastine, They are useful for treating the itching hydroxyzine, alimemazine, cyproheptadine, promethazine, A [1] and ketotifen.[4] Non-sedating antihistamines include that results from the release of histamine. -

Inhibitory Effect of Eslicarbazepine Acetate and S-Licarbazepine on 2 Nav1.5 Channels
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.24.059188; this version posted August 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Inhibitory effect of eslicarbazepine acetate and S-licarbazepine on 2 Nav1.5 channels 3 Theresa K. Leslie1, Lotte Brückner 1, Sangeeta Chawla1,2, William J. Brackenbury1,2* 4 1Department of Biology, University of York, Heslington, York, YO10 5DD, UK 5 2York Biomedical Research Institute, University of York, Heslington, York, YO10 5DD, UK 6 * Correspondence: Dr William J. Brackenbury, Department of Biology and York Biomedical 7 Research Institute, University of York, Wentworth Way, Heslington, York YO10 5DD, UK. Email: 8 [email protected]. Tel: +44 1904 328284. 9 Keywords: Anticonvulsant, cancer, epilepsy, eslicarbazepine acetate, Nav1.5, S-licarbazepine, 10 voltage-gated Na+ channel. 11 Abstract 12 Eslicarbazepine acetate (ESL) is a dibenzazepine anticonvulsant approved as adjunctive treatment for 13 partial-onset epileptic seizures. Following first pass hydrolysis of ESL, S-licarbazepine (S-Lic) 14 represents around 95 % of circulating active metabolites. S-Lic is the main enantiomer responsible 15 for anticonvulsant activity and this is proposed to be through the blockade of voltage-gated Na+ 16 channels (VGSCs). ESL and S-Lic both have a voltage-dependent inhibitory effect on the Na+ current 17 in N1E-115 neuroblastoma cells expressing neuronal VGSC subtypes including Nav1.1, Nav1.2, 18 Nav1.3, Nav1.6 and Nav1.7. -

Eslicarbazepine Acetate: a New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures
Drugs R D DOI 10.1007/s40268-017-0197-5 REVIEW ARTICLE Eslicarbazepine Acetate: A New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures 1 1 1 Graciana L. Galiana • Angela C. Gauthier • Richard H. Mattson Ó The Author(s) 2017. This article is an open access publication Abstract Eslicarbazepine acetate is a new anti-epileptic drug belonging to the dibenzazepine carboxamide family Key Points that is currently approved as adjunctive therapy and monotherapy for partial-onset (focal) seizures. The drug Eslicarbazepine acetate is an effective and safe enhances slow inactivation of voltage-gated sodium chan- treatment option for partial-onset seizures as nels and subsequently reduces the activity of rapidly firing adjunctive therapy and monotherapy. neurons. Eslicarbazepine acetate has few, but some, drug– drug interactions. It is a weak enzyme inducer and it Eslicarbazepine acetate improves upon its inhibits cytochrome P450 2C19, but it affects a smaller predecessors, carbamazepine and oxcarbazepine, by assortment of enzymes than carbamazepine. Clinical being available in a once-daily regimen, interacting studies using eslicarbazepine acetate as adjunctive treat- with a smaller range of drugs, and causing less side ment or monotherapy have demonstrated its efficacy in effects. patients with refractory or newly diagnosed focal seizures. The drug is generally well tolerated, and the most common side effects include dizziness, headache, and diplopia. One of the greatest strengths of eslicarbazepine acetate is its ability to be administered only once per day. Eslicar- 1 Introduction bazepine acetate has many advantages over older anti- epileptic drugs, and it should be strongly considered when Epilepsy is a common neurological disorder affecting over treating patients with partial-onset epilepsy. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Your Child's Emergency Allergy Pack with Antihistamine
Your Child’s Emergency Allergy Pack with Antihistamine Patient information Paediatric Department Watford General Hospital Hemel Hempstead Hospital If you need this leaflet in another language, large print, Braille or audio version, please call 01923 217 187 or email [email protected] Author Dr Ashley Reece Department Paediatrics Ratified Date / Review Date Feb 2021 / Feb 2024 Version Number / ID Number 40-1104-V1 Why does my child I need an Allergy Action Pack? An allergy action pan is a kit contianing evrything you need in case of an allergic reaction. Is my/ my child’s allergy severe? There is no such thing as a mild or severe allergy as reactions are always unpredicatble. However we do classify reactions as mild, moderate or severe. While it follows that a severe reaction makes the anxiety about the severity of the next reaction high, we cannot predict the severity of subsequent reactions. However there are some foods and some allergies which are assessed as having very low risk of a significant reaction. The way to manage your child with their allergy is to ensure you have the best possible safety net in case of an unforeseen reaction. Your doctor will give you a specific allergy plan (like the one to the right) which you should also give to your child’s school. Generally allergy is mananged by: You MUST avoid any foods which you know your child is allergic to. Avoidance Take care with labels and risk assess any new foods and when eating out in a restaurant or from a take-away. -
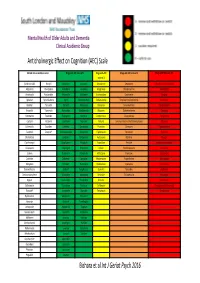
AEC Traffic Light System
Mental Health of Older Adults and Dementia Clinical Academic Group Anticholinergic Effect on Cognition (AEC) Scale Limited data so unable to score Drugs with AEC score of 0 Drugs with AEC Drugs with AEC score of 2 Drugs with AEC score of 3 score of 1 Alendronic Acid Ramipril Alprazolam Lovastatin Amiodarone Amantadine Alimemazine (trimeprazine) Allopurinol Rivaroxaban Amlodipine Lurasidone Aripiprazole Chlorphenamine Amitriptyline Anastrozole Rosuvastatin Amoxycillin Meloxicam Bromocriptine Desipramine Atropine Apixaban Spironolactone Aspirin Metoclopramide Carbamazepine Dicycloverine (dicyclomine) Benztropine Baclofen Tamoxifen Atenolol Metoprolol Citalopram Dimenhydrinate Chlorpromazine Bisoprolol Topiramate Atorvastatin Moclobemide Diazepam Diphenhydramine Clemastine Bumetanide Tizanidine Buproprion Morphine Domperidone Disopyramide Clomipramine Captopril Verapamil Cepahlexin Naproxen Fentanyl Levomepromazine (methotrimeprazine) Clozapine Carbimazole Zopiclone Cetirizine Omeprazole Fluoxetine Olanzapine Cyproheptadine Carvedilol Zotepine* Chlordiazepoxide Paracetamol Fluphenazine Paroxetine Dothiepin Chlortalidone Cimetidine Pantoprazole Hydroxyzine Pethidine Doxepin Clarithromycin Ciprofloxacin Pravastatin Iloperidone Pimozide Hyoscine hydrobromide Clonazepam Clopidogrel Propranolol Lithium Prochlorperazine Imipramine Codeine Darifenacin Rabeprazole Mirtazapine Promazine Lofepramine Colchicine Diclofenac Ranitidine Perphenazine Propantheline Nortriptyline Dabigatran Diltiazem Risperidone Prednisolone Quetiapine Orphenadrine Dexamethasone -

Eslicarbazepine Acetate for the Adjunctive Treatment of Partial-Onset Seizures with Or Without Secondary Generalisation in Patients Over 18 Years of Age
Effective Shared Care Agreement (ESCA) Eslicarbazepine acetate For the adjunctive treatment of partial-onset seizures with or without secondary generalisation in patients over 18 years of age. AREAS OF RESPONSIBILITY FOR THE SHARING OF CARE This shared care agreement outlines suggested ways in which the responsibilities for managing the prescribing of eslicarbazepine acetate for epileptic seizures can be shared between the specialist and general practitioner (GP). You are invited to participate however, if you do not feel confident to undertake this role, then you are not obliged to do so. In such an event, the total clinical responsibility for the patient for the diagnosed condition remains with the specialist. Sharing of care assumes communication between the specialist, GP and patient. The intention to share care will be explained to the patient by the specialist initiating treatment. It is important that patients are consulted about treatment and are in agreement with it. Patients with epilepsy are usually under regular specialist follow-up, which provides an opportunity to discuss drug therapy. The doctor who prescribes the medication legally assumes clinical responsibility for the drug and the consequences of its use. RESPONSIBILITIES and ROLES Specialist responsibilities 1. Confirm the diagnosis of epilepsy. 2. Confirm the patient has used oxcarbazepine (at maximum tolerated dose) or has documentation of intolerance. 3. Obtain approval via Trust’s DTC (or equivalent decision making body) before eslicarbazepine acetate is initiated. Please complete details on page 3. 4. Perform baseline assessment and periodic review of renal and hepatic function (as indicated for each patient). 5. Discuss the potential benefits, treatment side effects, and possible drug interactions with the patient. -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT (Invented name), film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol..............................................................................................................................500.00 mg Chlorphenamine maleate..............................................................................................................4.00 mg For one film-coated tablet Excipients with known effect: Carmoisine (E122) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Oblong purple film-coated tablet. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications This medicinal product is indicated for treatment, during the course of colds, rhinitis, rhinopharyngitis and flu-like conditions in adults and children aged over 15 years: of clear nasal discharge and watering of the eyes, of sneezing, of headaches and/or fever. 4.2 Posology and method of administration Posology Restricted to adults and children over the age of 15 years. Weight Dose per Administration Maximum daily dose (age) administration interval Adults and 1 tablet 4 hours 4 tablets children >50 kg i.e., i.e., (>15 years) 500 mg of paracetamol 2,000 mg of paracetamol 4 mg of chlorphenamine 16 mg of chlorphenamine Do not exceed the maximum posology of 4 tablets per 24 hours. Patients with renal failure In the event of renal failure and unless otherwise medically advised, it is recommended that the dose be reduced and that the minimum interval between the 2 doses be increased, based on the following table: 2 Creatinine Administration interval clearance ≥50 ml/min 4 hours 10–50 ml/min 6 hours <10 ml/min 8 hours The total dose of paracetamol should not exceed 3 g/day. Patients with hepatic failure In patients with active or compensated chronic hepatic disease, particularly those with hepatocellular insufficiency, chronic alcoholism, chronic malnutrition (low reserves of hepatic glutathione), and dehydration, the dose of paracetamol should not exceed 3 g/day. -

Pharmacokinetic and Pharmacodynamic Interactions Between Antiepileptics and Antidepressants Domenico Italiano University of Messina, Italy
University of Kentucky UKnowledge Psychiatry Faculty Publications Psychiatry 11-2014 Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants Domenico Italiano University of Messina, Italy Edoardo Spina University of Messina, Italy Jose de Leon University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/psychiatry_facpub Part of the Psychiatry and Psychology Commons Repository Citation Italiano, Domenico; Spina, Edoardo; and de Leon, Jose, "Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants" (2014). Psychiatry Faculty Publications. 40. https://uknowledge.uky.edu/psychiatry_facpub/40 This Article is brought to you for free and open access by the Psychiatry at UKnowledge. It has been accepted for inclusion in Psychiatry Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Pharmacokinetic and Pharmacodynamic Interactions between Antiepileptics and Antidepressants Notes/Citation Information Published in Expert Opinion on Drug Metabolism & Toxicology, v. 10, Issue 11, p. 1457-1489. © 2014 Taylor & Francis Group This is an Accepted Manuscript of an article published by Taylor & Francis Group in Expert Opinion on Drug Metabolism & Toxicology in Nov. 2014, available online: http://www.tandfonline.com/10.1517/ 17425255.2014.956081 Digital Object Identifier (DOI) http://dx.doi.org/10.1517/17425255.2014.956081 This article is available at UKnowledge: https://uknowledge.uky.edu/psychiatry_facpub/40 1 This is an Accepted Manuscript of an article published by Taylor & Francis Group in Expert Opinion on Drug Metabolism & Toxicology in Nov. -
![APTIOM (Eslicarbazepine Acetate) Is (S)-10-Acetoxy-10,11-Dihydro-5H Dibenz[B,F]Azepine-5-Carboxamide](https://docslib.b-cdn.net/cover/5440/aptiom-eslicarbazepine-acetate-is-s-10-acetoxy-10-11-dihydro-5h%C2%AD-dibenz-b-f-azepine-5-carboxamide-1265440.webp)
APTIOM (Eslicarbazepine Acetate) Is (S)-10-Acetoxy-10,11-Dihydro-5H Dibenz[B,F]Azepine-5-Carboxamide
HIGHLIGHTS OF PRESCRIBING INFORMATION Monitor and discontinue if another cause cannot be established. (5.2, 5.3, These highlights do not include all the information needed to use 5.4) APTIOM safely and effectively. See full prescribing information for • Hyponatremia: Monitor sodium levels in patients at risk or patients APTIOM. experiencing hyponatremia symptoms. (5.5) • Neurological Adverse Reactions: Monitor for dizziness, disturbance in gait APTIOM® (eslicarbazepine acetate) tablets, for oral use and coordination, somnolence, fatigue, cognitive dysfunction, and visual Initial U.S. Approval: 2013 changes. Use caution when driving or operating machinery. (5.6) • Withdrawal of APTIOM: Withdraw APTIOM gradually to minimize the ---------------------------RECENT MAJOR CHANGES------------------------- risk of increased seizure frequency and status epilepticus. (2.6, 5.7, 8.1) Indications and Usage (1) 9/2017 • Dosage and Administration (2) 9/2017 Drug Induced Liver Injury: Discontinue APTIOM in patients with jaundice Warnings and Precautions (5) 9/2017 or evidence of significant liver injury. (5.8) • Hematologic Adverse Reactions: Consider discontinuing. (5.10) ----------------------------INDICATIONS AND USAGE-------------------------- APTIOM is indicated for the treatment of partial-onset seizures in patients 4 ------------------------------ADVERSE REACTIONS------------------------------ years of age and older. (1) • Most common adverse reactions in adult patients receiving APTIOM (≥4% and ≥2% greater than placebo): dizziness, somnolence, nausea, headache, ----------------------DOSAGE AND ADMINISTRATION---------------------- diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. (6.1) • Adult Patients: The recommended initial dosage of APTIOM is 400 mg • Adverse reactions in pediatric patients are similar to those seen in adult once daily. For some patients, treatment may be initiated at 800 mg once patients. daily if the need for seizure reduction outweighs an increased risk of adverse reactions.