Your Child's Emergency Allergy Pack with Antihistamine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Potentially Inappropriate Prescriptions (Pips) for Older People (Modified from ‘STOPP/START 2’ O’Mahony Et Al 2014)
Appendix A: Potentially Inappropriate Prescriptions (PIPs) for older people (modified from ‘STOPP/START 2’ O’Mahony et al 2014) Consider holding (or deprescribing - consult with patient): 1. Any drug prescribed without an evidence-based clinical indication 2. Any drug prescribed beyond the recommended duration, where well-defined 3. Any duplicate drug class (optimise monotherapy) Avoid hazardous combinations e.g.: 1. The Triple Whammy: NSAID + ACE/ARB + diuretic in all ≥ 65 year olds (NHS Scotland 2015) 2. Sick Day Rules drugs: Metformin or ACEi/ARB or a diuretic or NSAID in ≥ 65 year olds presenting with dehydration and/or acute kidney injury (AKI) (NHS Scotland 2015) 3. Anticholinergic Burden (ACB): Any additional medicine with anticholinergic properties when already on an Anticholinergic/antimuscarinic (listed overleaf) in > 65 year olds (risk of falls, increased anticholinergic toxicity: confusion, agitation, acute glaucoma, urinary retention, constipation). The following are known to contribute to the ACB: Amantadine Antidepressants, tricyclic: Amitriptyline, Clomipramine, Dosulepin, Doxepin, Imipramine, Nortriptyline, Trimipramine and SSRIs: Fluoxetine, Paroxetine Antihistamines, first generation (sedating): Clemastine, Chlorphenamine, Cyproheptadine, Diphenhydramine/-hydrinate, Hydroxyzine, Promethazine; also Cetirizine, Loratidine Antipsychotics: especially Clozapine, Fluphenazine, Haloperidol, Olanzepine, and phenothiazines e.g. Prochlorperazine, Trifluoperazine Baclofen Carbamazepine Disopyramide Loperamide Oxcarbazepine Pethidine -

Chlorphenamine Maleate)
Package leaflet: Information for the patient Chlorphenamine 10 mg/ml Solution for Injection (Chlorphenamine Maleate) Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. − Keep this leaflet. You may need to read it again. − If you have any further questions, ask your doctor or nurse. − If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Chlorphenamine is and what it is used for 2. What you need to know before Chlorphenamine is given 3. How Chlorphenamine is given 4. Possible side effects 5. How to store Chlorphenamine 6. Contents of the pack and other information 1. What Chlorphenamine is and what it is used for Chlorphenamine 10 mg/ml Solution for Injection contains the active ingredient chlorphenamine maleate which is an antihistamine. Chlorphenamine is indicated in adults and children (aged 1 month to 18 years) for the treatment of acute allergic reactions. These medicines inhibit the release of histamine into the body that occurs during an allergic reaction. This product relieves some of the main symptoms of a severe allergic reaction. 2. What you need to know before Chlorphenamine is given You MUST NOT be given Chlorphenamine: if you are allergic to chlorphenamine maleate or any of the other ingredients of this medicine (listed in section 6) if you have had monoamine oxidase inhibitor (MAOI) antidepressive treatment within the past 14 days. Warnings and precautions Talk to your doctor or nurse before you are given this medicine if you: are being treated for an overactive thyroid or enlarged prostate gland have epilepsy, raised blood pressure within the eye or glaucoma, very high blood pressure, heart, liver, asthma or other chest diseases. -

Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj
Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj Nehad J. Ahmed1*, Menshawy A. Menshawy2 1Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia, 2Department of Medicinal chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia Abstract Aim: This study aims to illustrate the prescribing trends of antihistamines in the outpatient setting in Al-Kharj. Materials and Methods: This is a retrospective study that included the evaluation of antihistamines in the outpatient setting in a public hospital in Al-Kharj. The data were collected from the pharmacy-based computer system. Results: The total number of prescriptions that included antihistamines was 799. Most of the prescribed ORIGINAL ARTICLE ARTICLE ORIGINAL antihistamines were first-generation sedating antihistamines (chlorphenamine and diphenhydramine) (66.33%). About 63.20% of the prescribed antihistamines included chlorpheniramine followed by cetirizine (19.27%) and loratadine (14.39%). Conclusion: Antihistamines were prescribed commonly in the outpatient setting mainly first-generation sedating antihistamines. It is recommended to increase the awareness of health- care providers about the efficacy and the side effects of antihistamines and to encourage them to use these agents wisely. Key words: Antihistamines, outpatient, prescribing, trends INTRODUCTION In addition, antihistamines have been classified as sedating antihistamines (first-generation antihistamines) and non- ntihistamines are used in the sedating antihistamines (second-generation antihistamines).[4] management of allergic conditions. Sedating antihistamines include chlorphenamine, clemastine, They are useful for treating the itching hydroxyzine, alimemazine, cyproheptadine, promethazine, A [1] and ketotifen.[4] Non-sedating antihistamines include that results from the release of histamine. -

Cetirizine) – New Drug Approval
Quzyttir™ (cetirizine) – New drug approval • On October 4, 2019, the FDA approved TerSera Therapeutics’ Quzyttir (cetirizine) injection, for the treatment of acute urticaria in adults and children 6 months of age and older. — Quzyttir is not recommended in pediatric patients less than 6 years of age with impaired renal or hepatic function. • Quzyttir is the first FDA approved intravenous (IV) formulation of cetirizine. Oral formulations of cetirizine are available generically as prescription as well as well over-the-counter (OTC) (eg, Zyrtec®). — Prescription oral cetirizine is approved for perennial allergic rhinitis and chronic urticaria. — Zyrtec and other OTC products are approved for use to temporarily relieve symptoms due to hay fever or other upper respiratory allergies: runny nose, sneezing, itchy and watery eyes, and itching of the nose or throat. • The efficacy of Quzyttir was established in a randomized, active-controlled, double-blind, single-dose study in 262 adult patients with acute urticaria. Patients were treated with Quzyttir or diphenhydramine injection. The primary efficacy endpoint was the change from baseline in patient-rated pruritus score assessed 2 hours post treatment. The study was non-inferiority design with the pre-specified non- inferiority margin of 0.50 for the difference between treatment groups. — The effectiveness of Quzyttir was demonstrated to be non-inferior to the effectiveness of diphenhydramine. The mean change from baseline in patient-rated pruritus score was -1.61 and -1.50 for Quzyttir and diphenhydramine, respectively (adjusted difference: 0.06; 95% CI: - 0.28, 0.40). • The efficacy of Quzyttir for the treatment of acute urticaria down to 6 months of age is based on extrapolation of the efficacy of Quzyttir in adults with acute urticaria and supported by pharmacokinetic data with oral cetirizine hydrochloride in patients 6 months to 17 years of age. -

Cetirizine Hydrochloride) Tablets and Syrup for Oral Use
70-4573-00-5 ZYRTEC® (cetirizine hydrochloride) Tablets and Syrup For Oral Use DESCRIPTION Cetirizine hydrochloride, the active component of ZYRTEC® tablets and syrup, is an orally active and selective H1-receptor antagonist. The chemical name is (±) - [2- [4- [ (4- chlorophenyl)phenylmethyl] -1- piperazinyl] ethoxy]acetic acid, dihydrochloride. Cetirizine hydrochloride is a racemic compound with an empirical formula of C21H25ClN2O3•2HCl. The molecular weight is 461.82 and the chemical structure is shown below: Cl • CH - N N - CH2 - CH2 - O - CH2 - COOH 2HCl Cetirizine hydrochloride is a white, crystalline powder and is water soluble. ZYRTEC tablets are formulated as white, film-coated, rounded-off rectangular shaped tablets for oral administration and are available in 5 and 10 mg strengths. Inactive ingredients are: lactose; magnesium stearate; povidone; titanium dioxide; hydroxypropyl methylcellulose; polyethylene glycol; and corn starch. ZYRTEC syrup is a colorless to slightly yellow syrup containing cetirizine hydrochloride at a concentration of 1 mg/mL (5 mg/5 mL) for oral administration. The pH is between 4 and 5. The inactive ingredients of the syrup are: banana flavor; glacial acetic acid; glycerin; grape flavor; methylparaben; propylene glycol; propylparaben; sodium acetate; sugar syrup; and water. CLINICAL PHARMACOLOGY Mechanism of Actions: Cetirizine, a human metabolite of hydroxyzine, is an antihistamine; its principal effects are mediated via selective inhibition of peripheral H1 receptors. The antihistaminic activity of cetirizine has been clearly documented in a variety of animal and human models. In vivo and ex vivo animal models have shown negligible anticholinergic and antiserotonergic activity. In clinical studies, however, dry mouth was more common with cetirizine than with placebo. -
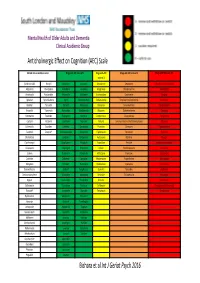
AEC Traffic Light System
Mental Health of Older Adults and Dementia Clinical Academic Group Anticholinergic Effect on Cognition (AEC) Scale Limited data so unable to score Drugs with AEC score of 0 Drugs with AEC Drugs with AEC score of 2 Drugs with AEC score of 3 score of 1 Alendronic Acid Ramipril Alprazolam Lovastatin Amiodarone Amantadine Alimemazine (trimeprazine) Allopurinol Rivaroxaban Amlodipine Lurasidone Aripiprazole Chlorphenamine Amitriptyline Anastrozole Rosuvastatin Amoxycillin Meloxicam Bromocriptine Desipramine Atropine Apixaban Spironolactone Aspirin Metoclopramide Carbamazepine Dicycloverine (dicyclomine) Benztropine Baclofen Tamoxifen Atenolol Metoprolol Citalopram Dimenhydrinate Chlorpromazine Bisoprolol Topiramate Atorvastatin Moclobemide Diazepam Diphenhydramine Clemastine Bumetanide Tizanidine Buproprion Morphine Domperidone Disopyramide Clomipramine Captopril Verapamil Cepahlexin Naproxen Fentanyl Levomepromazine (methotrimeprazine) Clozapine Carbimazole Zopiclone Cetirizine Omeprazole Fluoxetine Olanzapine Cyproheptadine Carvedilol Zotepine* Chlordiazepoxide Paracetamol Fluphenazine Paroxetine Dothiepin Chlortalidone Cimetidine Pantoprazole Hydroxyzine Pethidine Doxepin Clarithromycin Ciprofloxacin Pravastatin Iloperidone Pimozide Hyoscine hydrobromide Clonazepam Clopidogrel Propranolol Lithium Prochlorperazine Imipramine Codeine Darifenacin Rabeprazole Mirtazapine Promazine Lofepramine Colchicine Diclofenac Ranitidine Perphenazine Propantheline Nortriptyline Dabigatran Diltiazem Risperidone Prednisolone Quetiapine Orphenadrine Dexamethasone -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Antihistamines in the Treatment of Chronic Urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 a Del Cuvillo,6 J Mullol,7 J Sastre,8 a Valero5
Antihistamines in the treatment of chronic urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 A del Cuvillo,6 J Mullol,7 J Sastre,8 A Valero5 1 Service of Allergy, Hospital de Basurto, Bilbao, Spain 2 Department of Allergology, Clínica Universitaria de Navarra, Pamplona, Spain 3 Allergy Unit, Hospital La Plana, Villarreal (Castellón), Spain 4 Service of Immunoallergy, Hospital Clínico, Salamanca, Spain 5 Allergy Unit, Service of Pneumology and Respiratory Allergy, Hospital Clínic (ICT), Barcelona, Spain 6 Clínica Dr. Lobatón, Cádiz, Spain 7 Rhinology Unit, ENT Service (ICEMEQ), Hospital Clínic, Barcelona, Spain 8 Service of Allergy, Fundación Jiménez Díaz, Madrid, Spain ■ Summary Chronic urticaria is highly prevalent in the general population, and while there are multiple treatments for the disorder, the results obtained are not completely satisfactory. The second-generation H1 antihistamines remain the symptomatic treatment option of choice. Depending on the different pharmacokinetics and H1 receptor affi nity of each drug substance, different concentrations in skin can be expected, together with different effi cacy in relation to the histamine-induced wheal inhibition test - though this does not necessarily have repercussions upon clinical response. The antiinfl ammatory properties of the H1 antihistamines could be of relevance in chronic urticaria, though it is not clear to what degree they infl uence the fi nal therapeutic result. Before moving on to another therapeutic level, the advisability of antihistamine dose escalation should be considered, involving increments even above those approved in the Summary of Product Characteristics. Physical urticaria, when manifesting isolatedly, tends to respond well to H1 antihistamines, with the exception of genuine solar urticaria and delayed pressure urticaria. -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT (Invented name), film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol..............................................................................................................................500.00 mg Chlorphenamine maleate..............................................................................................................4.00 mg For one film-coated tablet Excipients with known effect: Carmoisine (E122) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Oblong purple film-coated tablet. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications This medicinal product is indicated for treatment, during the course of colds, rhinitis, rhinopharyngitis and flu-like conditions in adults and children aged over 15 years: of clear nasal discharge and watering of the eyes, of sneezing, of headaches and/or fever. 4.2 Posology and method of administration Posology Restricted to adults and children over the age of 15 years. Weight Dose per Administration Maximum daily dose (age) administration interval Adults and 1 tablet 4 hours 4 tablets children >50 kg i.e., i.e., (>15 years) 500 mg of paracetamol 2,000 mg of paracetamol 4 mg of chlorphenamine 16 mg of chlorphenamine Do not exceed the maximum posology of 4 tablets per 24 hours. Patients with renal failure In the event of renal failure and unless otherwise medically advised, it is recommended that the dose be reduced and that the minimum interval between the 2 doses be increased, based on the following table: 2 Creatinine Administration interval clearance ≥50 ml/min 4 hours 10–50 ml/min 6 hours <10 ml/min 8 hours The total dose of paracetamol should not exceed 3 g/day. Patients with hepatic failure In patients with active or compensated chronic hepatic disease, particularly those with hepatocellular insufficiency, chronic alcoholism, chronic malnutrition (low reserves of hepatic glutathione), and dehydration, the dose of paracetamol should not exceed 3 g/day. -

How to Recognise and Manage Mild to Moderate Allergic Reactions in Children Information for Parents and Carers Contents Page What Is an Allergic Reaction? 2
Children’s Allergy Clinic How to recognise and manage mild to moderate allergic reactions in children Information for parents and carers Contents Page What is an allergic reaction? 2 What can cause allergic reactions? 3 How to avoid contact with allergens 4 Signs and symptoms 6 Action plan 7 Nurseries, child-minders, schools/activity groups 9 Further information 11 What is an allergic reaction? An allergic reaction happens when the body’s immune system over-reacts to contact with normally harmless substances. An allergic person’s immune system treats certain substances as threats and releases substances such as histamines to defend the body against them. The release of histamine can cause the body to produce a range of mild to severe symptoms. An allergic response can develop after touching, swallowing, tasting, eating or breathing-in a particular substance. page 2 What can cause allergic reactions? Foods For example: • nuts (especially peanuts) • fish and shellfish • eggs and milk. Most allergic reactions to food occur immediately after swallowing, although some can occur up to several hours afterwards. Food allergies are more common in families who have other allergic conditions such as asthma, eczema and hay fever. Rarely, people have an allergic reaction to fruit, vegetables and legumes. Legumes include pulses, beans, peas and lentils. Peanuts are also part of the legume family. Insect stings • Reaction to an insect sting is immediate (within 30 minutes). Natural rubber latex Some common sources of latex are: • balloons • rubber bands • carpet backing • furniture filling • medical or dental items such as catheters, gloves, disposable items. Medicines Medication rarely causes a severe allergic reaction in children. -

Thermodynamic Investigation of Promethazine, Loratadine, Cetirizine and Buclizine As Antihistamine Drugs; Monte Carlo and Semi-Empirical Studies
ISSN-E 1995-9516 Universidad Nacional de Ingeniería COPYRIGHT © (UNI). TODOS LOS DERECHOS RESERVADOS http://revistas.uni.edu.ni/index.php/Nexo https://doi.org/10.5377/nexo.v33i01.10050 Vol. 33, No. 01, pp. 94-108/Junio 2020 THERMODYNAMIC INVESTIGATION OF PROMETHAZINE, LORATADINE, CETIRIZINE AND BUCLIZINE AS ANTIHISTAMINE DRUGS; MONTE CARLO AND SEMI-EMPIRICAL STUDIES INVESTIGACIÓN TERMODINÁMICA DE PROMETAZINA, LORATADINA, CETIRIZINA Y BUCLIZINA COMO MEDICAMENTOS ANTIHISTAMÍNICOS; MONTE CARLO Y ESTUDIOS SEMI-EMPÍRICOS Mina Zakeri1, Majid Monajjemi2*, Ali Ebrahimi3 1Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Department of Chemical Engineering, Central Tehran branch, Islamic Azad University Tehran, Iran 3Department of chemistry, Computational Quantum Chemistry Laboratory, University of Sistan and Baluchestan, Iran *[email protected] (recibido/received: 14-diciembre-2019; aceptado/accepted: 21-febrero-2020) ABSTRACT In this article, we discussed about four antihistamine drug called promethazine, loratadine, cetirizine and buclizine. Promethazine in this list is the only one in first generation antihistamine classification with CNS sedation effect and the other three belongs to second generation antihistamine group which are non- sedation and used to treat in many different anti-allergenic fields. In the following we optimized potential, kinetic and total energy of these molecules at body temperature (310 k˚) and environment temperature (298 k ˚) using Mont Carlo method in Amber force field in 500 ns. The quantum mechanics calculations and molecular structure of these molecules investigated using B3LYP level of theory with 6-31 G (d) as a basis set. Theoretical computations were performed to study thermodynamic parameters and frequency analysis. Electronic, thermal, zero point and gibs free energy and enthalpy were estimated in frequency analysis. -

Cetirizine Hcl 5 Mg
NDC 0228-1196-55 Active ingredient (in each tablet) Purpose Cetirizine HCl 5 mg . Antihistamine Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: N runny nose N sneezing N itchy, watery eyes N itching of the nose or Cetirizine throat Hydrochloride Warnings Do not use if you have ever had an allergic Tablets 5 mg reaction to this product or any of its ingredients or to an Antihistamine antihistamine containing hydroxyzine. Rev. 11/07 Allergy Mfd by: Shasun Chemical & Drugs Limited, Indoor & Outdoor Allergies Unit II, R.S. No.: 32, 33, & 34, PIMS Road, Periyakalapet, Pondicherry – 605014. India 100 tablets - 5 mg each or missing. on bottle is broken For: Actavis Elizabeth LLC, 200 Elmora Avenue PEEL Do not use if imprinted foil inner seal foil Do not use if imprinted Elizabeth, NJ 07207 USA HERE Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose. Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives. When using this product N drowsiness may occur N avoid alcoholic drinks N alcohol, sedatives, and tranquilizers may increase drowsiness N be careful when driving a motor vehicle or operating machinery. Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away. If pregnant or breast-feeding: N if breast-feeding: not recommended N if pregnant: ask a health professional before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.