Committee for Veterinary Medicinal Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Potentially Inappropriate Prescriptions (Pips) for Older People (Modified from ‘STOPP/START 2’ O’Mahony Et Al 2014)
Appendix A: Potentially Inappropriate Prescriptions (PIPs) for older people (modified from ‘STOPP/START 2’ O’Mahony et al 2014) Consider holding (or deprescribing - consult with patient): 1. Any drug prescribed without an evidence-based clinical indication 2. Any drug prescribed beyond the recommended duration, where well-defined 3. Any duplicate drug class (optimise monotherapy) Avoid hazardous combinations e.g.: 1. The Triple Whammy: NSAID + ACE/ARB + diuretic in all ≥ 65 year olds (NHS Scotland 2015) 2. Sick Day Rules drugs: Metformin or ACEi/ARB or a diuretic or NSAID in ≥ 65 year olds presenting with dehydration and/or acute kidney injury (AKI) (NHS Scotland 2015) 3. Anticholinergic Burden (ACB): Any additional medicine with anticholinergic properties when already on an Anticholinergic/antimuscarinic (listed overleaf) in > 65 year olds (risk of falls, increased anticholinergic toxicity: confusion, agitation, acute glaucoma, urinary retention, constipation). The following are known to contribute to the ACB: Amantadine Antidepressants, tricyclic: Amitriptyline, Clomipramine, Dosulepin, Doxepin, Imipramine, Nortriptyline, Trimipramine and SSRIs: Fluoxetine, Paroxetine Antihistamines, first generation (sedating): Clemastine, Chlorphenamine, Cyproheptadine, Diphenhydramine/-hydrinate, Hydroxyzine, Promethazine; also Cetirizine, Loratidine Antipsychotics: especially Clozapine, Fluphenazine, Haloperidol, Olanzepine, and phenothiazines e.g. Prochlorperazine, Trifluoperazine Baclofen Carbamazepine Disopyramide Loperamide Oxcarbazepine Pethidine -

Chlorphenamine Maleate)
Package leaflet: Information for the patient Chlorphenamine 10 mg/ml Solution for Injection (Chlorphenamine Maleate) Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. − Keep this leaflet. You may need to read it again. − If you have any further questions, ask your doctor or nurse. − If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Chlorphenamine is and what it is used for 2. What you need to know before Chlorphenamine is given 3. How Chlorphenamine is given 4. Possible side effects 5. How to store Chlorphenamine 6. Contents of the pack and other information 1. What Chlorphenamine is and what it is used for Chlorphenamine 10 mg/ml Solution for Injection contains the active ingredient chlorphenamine maleate which is an antihistamine. Chlorphenamine is indicated in adults and children (aged 1 month to 18 years) for the treatment of acute allergic reactions. These medicines inhibit the release of histamine into the body that occurs during an allergic reaction. This product relieves some of the main symptoms of a severe allergic reaction. 2. What you need to know before Chlorphenamine is given You MUST NOT be given Chlorphenamine: if you are allergic to chlorphenamine maleate or any of the other ingredients of this medicine (listed in section 6) if you have had monoamine oxidase inhibitor (MAOI) antidepressive treatment within the past 14 days. Warnings and precautions Talk to your doctor or nurse before you are given this medicine if you: are being treated for an overactive thyroid or enlarged prostate gland have epilepsy, raised blood pressure within the eye or glaucoma, very high blood pressure, heart, liver, asthma or other chest diseases. -

Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj
Prescribing Trends of Antihistamines in the Outpatient Setting in Al-Kharj Nehad J. Ahmed1*, Menshawy A. Menshawy2 1Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia, 2Department of Medicinal chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia Abstract Aim: This study aims to illustrate the prescribing trends of antihistamines in the outpatient setting in Al-Kharj. Materials and Methods: This is a retrospective study that included the evaluation of antihistamines in the outpatient setting in a public hospital in Al-Kharj. The data were collected from the pharmacy-based computer system. Results: The total number of prescriptions that included antihistamines was 799. Most of the prescribed ORIGINAL ARTICLE ARTICLE ORIGINAL antihistamines were first-generation sedating antihistamines (chlorphenamine and diphenhydramine) (66.33%). About 63.20% of the prescribed antihistamines included chlorpheniramine followed by cetirizine (19.27%) and loratadine (14.39%). Conclusion: Antihistamines were prescribed commonly in the outpatient setting mainly first-generation sedating antihistamines. It is recommended to increase the awareness of health- care providers about the efficacy and the side effects of antihistamines and to encourage them to use these agents wisely. Key words: Antihistamines, outpatient, prescribing, trends INTRODUCTION In addition, antihistamines have been classified as sedating antihistamines (first-generation antihistamines) and non- ntihistamines are used in the sedating antihistamines (second-generation antihistamines).[4] management of allergic conditions. Sedating antihistamines include chlorphenamine, clemastine, They are useful for treating the itching hydroxyzine, alimemazine, cyproheptadine, promethazine, A [1] and ketotifen.[4] Non-sedating antihistamines include that results from the release of histamine. -

Your Child's Emergency Allergy Pack with Antihistamine
Your Child’s Emergency Allergy Pack with Antihistamine Patient information Paediatric Department Watford General Hospital Hemel Hempstead Hospital If you need this leaflet in another language, large print, Braille or audio version, please call 01923 217 187 or email [email protected] Author Dr Ashley Reece Department Paediatrics Ratified Date / Review Date Feb 2021 / Feb 2024 Version Number / ID Number 40-1104-V1 Why does my child I need an Allergy Action Pack? An allergy action pan is a kit contianing evrything you need in case of an allergic reaction. Is my/ my child’s allergy severe? There is no such thing as a mild or severe allergy as reactions are always unpredicatble. However we do classify reactions as mild, moderate or severe. While it follows that a severe reaction makes the anxiety about the severity of the next reaction high, we cannot predict the severity of subsequent reactions. However there are some foods and some allergies which are assessed as having very low risk of a significant reaction. The way to manage your child with their allergy is to ensure you have the best possible safety net in case of an unforeseen reaction. Your doctor will give you a specific allergy plan (like the one to the right) which you should also give to your child’s school. Generally allergy is mananged by: You MUST avoid any foods which you know your child is allergic to. Avoidance Take care with labels and risk assess any new foods and when eating out in a restaurant or from a take-away. -
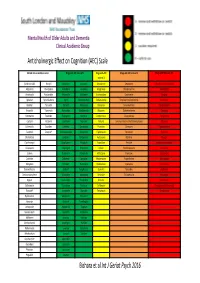
AEC Traffic Light System
Mental Health of Older Adults and Dementia Clinical Academic Group Anticholinergic Effect on Cognition (AEC) Scale Limited data so unable to score Drugs with AEC score of 0 Drugs with AEC Drugs with AEC score of 2 Drugs with AEC score of 3 score of 1 Alendronic Acid Ramipril Alprazolam Lovastatin Amiodarone Amantadine Alimemazine (trimeprazine) Allopurinol Rivaroxaban Amlodipine Lurasidone Aripiprazole Chlorphenamine Amitriptyline Anastrozole Rosuvastatin Amoxycillin Meloxicam Bromocriptine Desipramine Atropine Apixaban Spironolactone Aspirin Metoclopramide Carbamazepine Dicycloverine (dicyclomine) Benztropine Baclofen Tamoxifen Atenolol Metoprolol Citalopram Dimenhydrinate Chlorpromazine Bisoprolol Topiramate Atorvastatin Moclobemide Diazepam Diphenhydramine Clemastine Bumetanide Tizanidine Buproprion Morphine Domperidone Disopyramide Clomipramine Captopril Verapamil Cepahlexin Naproxen Fentanyl Levomepromazine (methotrimeprazine) Clozapine Carbimazole Zopiclone Cetirizine Omeprazole Fluoxetine Olanzapine Cyproheptadine Carvedilol Zotepine* Chlordiazepoxide Paracetamol Fluphenazine Paroxetine Dothiepin Chlortalidone Cimetidine Pantoprazole Hydroxyzine Pethidine Doxepin Clarithromycin Ciprofloxacin Pravastatin Iloperidone Pimozide Hyoscine hydrobromide Clonazepam Clopidogrel Propranolol Lithium Prochlorperazine Imipramine Codeine Darifenacin Rabeprazole Mirtazapine Promazine Lofepramine Colchicine Diclofenac Ranitidine Perphenazine Propantheline Nortriptyline Dabigatran Diltiazem Risperidone Prednisolone Quetiapine Orphenadrine Dexamethasone -

Summary of Product Characteristics
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT (Invented name), film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol..............................................................................................................................500.00 mg Chlorphenamine maleate..............................................................................................................4.00 mg For one film-coated tablet Excipients with known effect: Carmoisine (E122) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Oblong purple film-coated tablet. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications This medicinal product is indicated for treatment, during the course of colds, rhinitis, rhinopharyngitis and flu-like conditions in adults and children aged over 15 years: of clear nasal discharge and watering of the eyes, of sneezing, of headaches and/or fever. 4.2 Posology and method of administration Posology Restricted to adults and children over the age of 15 years. Weight Dose per Administration Maximum daily dose (age) administration interval Adults and 1 tablet 4 hours 4 tablets children >50 kg i.e., i.e., (>15 years) 500 mg of paracetamol 2,000 mg of paracetamol 4 mg of chlorphenamine 16 mg of chlorphenamine Do not exceed the maximum posology of 4 tablets per 24 hours. Patients with renal failure In the event of renal failure and unless otherwise medically advised, it is recommended that the dose be reduced and that the minimum interval between the 2 doses be increased, based on the following table: 2 Creatinine Administration interval clearance ≥50 ml/min 4 hours 10–50 ml/min 6 hours <10 ml/min 8 hours The total dose of paracetamol should not exceed 3 g/day. Patients with hepatic failure In patients with active or compensated chronic hepatic disease, particularly those with hepatocellular insufficiency, chronic alcoholism, chronic malnutrition (low reserves of hepatic glutathione), and dehydration, the dose of paracetamol should not exceed 3 g/day. -

Appendix 1 BNF Codes of Drugs Lists Used to Define Exposure Groups
Appendix 1 BNF Codes of drugs lists used to define exposure groups. Anticholinergic antipsychotics d411. Chlorpromazine d412. Chlorpromazine d413. Chlorpromazine d414. Chlorpromazine d415. Chlorpromazine d41a. Chlorpromazine d41b. Chlorpromazine d41c. Chlorpromazine d41d. Chlorpromazine d4b1. Perphenazine d4e1. PROMAZINE d4ex. PROMAZINE d4g.. Thioridazine d4g1. Thioridazine d4g2. Thioridazine d4g3. Thioridazine d4g5. Thioridazine d4g7. Thioridazine d4gp. Thioridazine d4gt. Thioridazine d4gu. Thioridazine d4gv. Thioridazine d4gw. Thioridazine d4gz. Thioridazine d4h.. Trifluoperazine d4h1. Trifluoperazine d4h2. Trifluoperazine d4h3. Trifluoperazine d4h4. Trifluoperazine d4hs. Trifluoperazine d4ht. Trifluoperazine d4hu. Trifluoperazine d4hx. Trifluoperazine d4l2. CLOZAPINE d4r1. OLANZAPINE d4r3. OLANZAPINE d4r7. OLANZAPINE d4s1. QUETIAPINE d4s2. QUETIAPINE d4s3. QUETIAPINE d4s5. QUETIAPINE d4ss. QUETIAPINE d4sx. QUETIAPINE Tricyclic antidepressants d7... d71.. Amitriptyline d711. Amitriptyline d712. Amitriptyline d713. Amitriptyline d719. Amitriptyline d71a. Amitriptyline d71b. Amitriptyline d71c. Amitriptyline d71d. Amitriptyline d71e. Amitriptyline d71f. Amitriptyline d71u. Amitriptyline d71v. Amitriptyline d71w. Amitriptyline d71y. Amitriptyline d71z. Amitriptyline d73.. Clomipramine d731. Clomipramine d732. Clomipramine d733. Clomipramine d736. Clomipramine d73s. Clomipramine d73t. Clomipramine d73u. Clomipramine d73v. Clomipramine d73w. Clomipramine d73z. Clomipramine d75.. DOSULEPIN d751. DOSULEPIN d752. DOSULEPIN d755. DOSULEPIN d756. -

PIL Pharmagrip Capsules HK
Package leaflet: Information for the user pharmagrip capsules Paracetamol/Phenylephrine hydrochloride/Chlorphenamine maleate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you. - Keep this leaflet. You may need to read it again. - Ask your pharmacist if you need more information or advice. - If you get side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. - You must talk to a doctor if you do not feel better or if you feel worse or if the fever persists for more than 3 days or the pain lasts for more than 5 days (2 days in the case of sore throat). What is in this leaflet: 1. What pharmagrip capsules is and what it is used for 2. What you need to know before you take pharmagrip capsules 3. How to take pharmagrip capsules 4. Possible side effects 5. How to store pharmagrip capsules 6. Contents of the pack and other information 1. What pharmagrip capsules is and what it is used for pharmagrip capsules is a combination of paracetamol (an analgesic that reduces pain and fever), chlorphenamine (an antihistamine that relieves nasal secretion) and phenylephrine (which acts reducing nasal congestion). This medicine is indicated for relief of the symptoms of cold or flu conditions associated with mild or moderate pain, fever and nasal congestion and secretion in adults and adolescents over 12 years of age. -

West Essex CCG Anticholinergic Side-Effects and Prescribing Guidance
Anticholinergic side-effects and prescribing guidance . Anticholinergic (antimuscarinic) medications: associated with increased risks of impaired cognition and falls in patients over the age of 65 years. Recent research also points to a link to mortality increasing with the number and potency of anticholinergic agents prescribed. Anticholinergic Syndrome: is a state of confusion with characteristic features related to dysfunction of the autonomic parasympathetic (cholinergic) nervous system. Symptoms classified into systemic and CNS manifestations: o Systemic (peripheral) symptoms: Blurred vision, photophobia, non-reactive mydriasis, loss of accommodation response, flushed and dry skin, dry mouth, tachycardia, hypertension and fever. Gastrointestinal and urinary motility are frequently reduced o CNS symptoms: Delirium, agitation, disorientation, and visual hallucinations. Ataxia, choreoathetosis, myoclonus and seizures may also occur without peripheral symptoms. Medication Issues: several commonly prescribed medications that may not be thought of as anticholinergic have significant anticholinergic effects, which when taken with known anticholinergic medication can increase the risk of adverse effects. Many medication groups e.g. antihistamines, tricyclic antidepressants, drugs for asthma and COPD, cold preparations, hyoscine have varying degrees of anticholinergic activity and have the potential to cause Anticholinergic Syndrome . Clinicians should be aware of the risk for chronic anticholinergic toxicity and the fact that not all the symptoms may manifest in patients and if they do suffer some symptoms they could be wrongly attributed to another diagnosis Evidence . A study of patients over 65 found that 20% of participants who scored four or more had died by the end of the two year study period compared with 7% of patients with a score of zero. -

EMERGENCY TREATMENT of ANAPHYLACTIC REACTIONS Resuscitation Council (UK)
Resuscitation Council (UK) Emergency treatment of anaphylactic reactions Guidelines for healthcare providers Working Group of the Resuscitation Council (UK) January 2008 Areas covered by Annotated with links to NICE guidance July 2012 NICE Clinical Guidance CG134 are Review Date: 2016 highlighted in the text with a pink sidebar, which is a web link to the NICE CG134 Published by the Resuscitation Council (UK) NICE guidance web page 5th Floor, Tavistock House North Tavistock Square London WC1H 9HR Tel: 020 7388 4678 Fax: 020 7383 0773 E-mail: [email protected] Website: www.resus.org.uk Registered charity no. 286360 Copyright Resuscitation Council (UK) No part of this publication may be reproduced without the written permission of the Resuscitation Council (UK). Resuscitation Council (UK) Members of the Working Group Jasmeet Soar – Co-chair Working Group, Vice Chair Resuscitation Council (UK) Richard Pumphrey – Co-chair Working Group, Royal College of Pathologists Andrew Cant – Royal College of Paediatrics and Child Health Sue Clarke – Anaphylaxis Campaign Allison Corbett – British National Formulary Peter Dawson – Royal College of Radiologists Pamela Ewan – British Society for Allergy and Clinical Immunology Bernard Foëx – College of Emergency Medicine David Gabbott – Executive Committee Member Resuscitation Council (UK) Matt Griffiths – Royal College of Nursing Judith Hall – Royal College of Anaesthetists Nigel Harper – Association of Anaesthetists of Great Britain & Ireland Fiona Jewkes – Royal College of General Practitioners, -

Myasthenia Gravis Or Lambert-Eaton Myasthenia Syndrome – Medicines That May Affect Patients
CLINICAL GUIDELINE Myasthenia Gravis or Lambert-Eaton Myasthenia Syndrome – Medicines that may affect patients A guideline is intended to assist healthcare professionals in the choice of disease-specific treatments. Clinical judgement should be exercised on the applicability of any guideline, influenced by individual patient characteristics. Clinicians should be mindful of the potential for harmful polypharmacy and increased susceptibility to adverse drug reactions in patients with multiple morbidities or frailty. If, after discussion with the patient or carer, there are good reasons for not following a guideline, it is good practice to record these and communicate them to others involved in the care of the patient. Version Number: 1 Does this version include n/a changes to clinical advice: Date Approved: 11th July 2018 Date of Next Review: 30th July 2021 Lead Author: Lesley Murray Approval Group: Medicines Utilisation Subcommittee of ADTC Important Note: The Intranet version of this document is the only version that is maintained. Any printed copies should therefore be viewed as ‘Uncontrolled’ and as such, may not necessarily contain the latest updates and amendments. Information for healthcare professionals Medicines that may affect patients with Myasthenia Gravis or Lambert-Eaton Myasthenic Syndrome There are certain medicines that have been reported to worsen or induce myasthenia gravis (MG), often by increasing muscular weakness, and should be used with caution in patients with this condition. The list of medicines in table 1 has been compiled to assist prescribers in the decision making process when prescribing medicines for patients with myasthenia gravis. The medicines in this list have been classed according to those which should be: ▲ ▲Absolutely contraindicated ▲ Avoided Used with caution Probably safe with patient monitoring. -

Allergy Skin Testing Instructions
2073 N. Clybourn Ave, Chicago, IL 60614 T 773.665.4016 / F 773.360.6200 ALLERGY SKIN TESTING INSTRUCTIONS Allergy skin testing provides a fast, safe and reliable means for identifying allergic sensitivities to inhalant allergens (e.g., pollens, molds, dust mites and animal danders) and is also used sometimes to diagnose allergic sensitivities to foods and sometimes antibiotics and insect stings. The information obtained from allergy testing provides guidance for avoidance of allergens and potential medication choices. Test results may also be used to formulate allergy shot extracts. In order to make your allergy testing appointment as productive as possible, we ask that you review the following instructions prior to your appointment: 1. Although the testing itself may be completed in one hour or less, additional time may be needed to discuss results, allergy avoidance measures, medications or allergy shot treatments. 2. Wear a shirt or blouse, which can be removed easily. Prick skin testing is performed using the Multitest™ device applied to the back. 3. Many medications interfere with allergy skin testing and many do not. Below you will find a list of both. Those that do interfere should be discontinued in the time specified. If you have a medical condition or severe allergic symptoms which might worsen without medications, please consult us prior to stopping these medications. We can often find a way to work around such problems. If you have forgotten to stop these medications by the specified time, please consult one of our nurses to determine whether or not you need to reschedule your allergy testing appointment.