10.1. CRYSTALLOGRAPHIC and NONCRYSTALLOGRAPHIC POINT GROUPS Table 10.1.1.2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Final Poster
Associating Finite Groups with Dessins d’Enfants Luis Baeza, Edwin Baeza, Conner Lawrence, and Chenkai Wang Abstract Platonic Solids Rotation Group Dn: Regular Convex Polygon Approach Each finite, connected planar graph has an automorphism group G;such Following Magot and Zvonkin, reduce to easier cases using “hypermaps” permutations can be extended to automorphisms of the Riemann sphere φ : P1(C) P1(C), then composing β = φ f where S 2(R) P1(C). In 1984, Alexander Grothendieck, inspired by a result of f : 1( ) ! 1( )isaBely˘ımapasafunctionofeither◦ zn or ' P C P C Gennadi˘ıBely˘ıfrom 1979, constructed a finite, connected planar graph 4 zn/(zn +1)! 2 such that Aut(f ) Z or Aut(f ) D ,respectively. ' n ' n ∆β via certain rational functions β(z)=p(z)/q(z)bylookingatthe inverse image of the interval from 0 to 1. The automorphisms of such a Hypermaps: Rotation Group Zn graph can be identified with the Galois group Aut(β)oftheassociated 1 1 rational function β : P (C) P (C). In this project, we investigate how Rigid Rotations of the Platonic Solids I Wheel/Pyramids (J1, J2) ! w 3 (w +8) restrictive Grothendieck’s concept of a Dessin d’Enfant is in generating all n 2 I φ(w)= 1 1 z +1 64 (w 1) automorphisms of planar graphs. We discuss the rigid rotations of the We have an action : PSL2(C) P (C) P (C). β(z)= : v = n + n, e =2 n, f =2 − n ◦ ⇥ 2 !n 2 4 zn · Platonic solids (the tetrahedron, cube, octahedron, icosahedron, and I Zn = r r =1 and Dn = r, s s = r =(sr) =1 are the rigid I Cupola (J3, J4, J5) dodecahedron), the Archimedean solids, the Catalan solids, and the rotations of the regular convex polygons,with 4w 4(w 2 20w +105)3 I φ(w)= − ⌦ ↵ ⌦ 1 ↵ Rotation Group A4: Tetrahedron 3 2 Johnson solids via explicit Bely˘ımaps. -

The Conway Space-Filling
Symmetry: Art and Science Buenos Aires Congress, 2007 THE CONWAY SPACE-FILLING MICHAEL LONGUET-HIGGINS Name: Michael S. Longuet-Higgins, Mathematician and Oceanographer, (b. Lenham, England, 1925). Address: Institute for Nonlinear Science, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92037-0402, U.S.A. Email: [email protected] Fields of interest: Fluid dynamics, ocean waves and currents, geophysics, underwater sound, projective geometry, polyhedra, mathematical toys. Awards: Rayleigh Prize for Mathematics, Cambridge University, 1950; Hon.D. Tech., Technical University of Denmark, 1979; Hon. LL.D., University of Glasgow, Scotland, 1979; Fellow of the American Geophysical Union, 1981; Sverdrup Gold Medal of the American Meteorological Society, 1983; International Coastal Engineering Award of the American Society of Civil Engineers, 1984; Oceanography Award of the Society for Underwater Technology, 1990; Honorary Fellow of the Acoustical Society of America, 2002. Publications: “Uniform polyhedra” (with H.S.M. Coxeter and J.C.P. Miller (1954). Phil. Trans. R. Soc. Lond. A 246, 401-450. “Some Mathematical Toys” (film), (1963). British Association Meeting, Aberdeen, Scotland; “Clifford’s chain and its analogues, in relation to the higher polytopes,” (1972). Proc. R. Soc. Lond. A 330, 443-466. “Inversive properties of the plane n-line, and a symmetric figure of 2x5 points on a quadric,” (1976). J. Lond. Math. Soc. 12, 206-212; Part II (with C.F. Parry) (1979) J. Lond. Math. Soc. 19, 541-560; “Nested triacontahedral shells, or How to grow a quasi-crystal,” (2003) Math. Intelligencer 25, 25-43. Abstract: A remarkable new space-filling, with an unusual symmetry, was recently discovered by John H. -

Computational Design Framework 3D Graphic Statics
Computational Design Framework for 3D Graphic Statics 3D Graphic for Computational Design Framework Computational Design Framework for 3D Graphic Statics Juney Lee Juney Lee Juney ETH Zurich • PhD Dissertation No. 25526 Diss. ETH No. 25526 DOI: 10.3929/ethz-b-000331210 Computational Design Framework for 3D Graphic Statics A thesis submitted to attain the degree of Doctor of Sciences of ETH Zurich (Dr. sc. ETH Zurich) presented by Juney Lee 2015 ITA Architecture & Technology Fellow Supervisor Prof. Dr. Philippe Block Technical supervisor Dr. Tom Van Mele Co-advisors Hon. D.Sc. William F. Baker Prof. Allan McRobie PhD defended on October 10th, 2018 Degree confirmed at the Department Conference on December 5th, 2018 Printed in June, 2019 For my parents who made me, for Dahmi who raised me, and for Seung-Jin who completed me. Acknowledgements I am forever indebted to the Block Research Group, which is truly greater than the sum of its diverse and talented individuals. The camaraderie, respect and support that every member of the group has for one another were paramount to the completion of this dissertation. I sincerely thank the current and former members of the group who accompanied me through this journey from close and afar. I will cherish the friendships I have made within the group for the rest of my life. I am tremendously thankful to the two leaders of the Block Research Group, Prof. Dr. Philippe Block and Dr. Tom Van Mele. This dissertation would not have been possible without my advisor Prof. Block and his relentless enthusiasm, creative vision and inspiring mentorship. -

Glide and Screw
Space Groups •The 32 crystallographic point groups, whose operation have at least one point unchanged, are sufficient for the description of finite, macroscopic objects. •However since ideal crystals extend indefinitely in all directions, we must also include translations (the Bravais lattices) in our description of symmetry. Space groups: formed when combining a point symmetry group with a set of lattice translation vectors (the Bravais lattices), i.e. self-consistent set of symmetry operations acting on a Bravais lattice. (Space group lattice types and translations have no meaning in point group symmetry.) Space group numbers for all the crystal structures we have discussed this semester, and then some, are listed in DeGraef and Rohrer books and pdf. document on structures and AFLOW website, e.g. ZnS (zincblende) belongs to SG # 216: F43m) Class21/1 Screw Axes •The combination of point group symmetries and translations also leads to two additional operators known as glide and screw. •The screw operation is a combination of a rotation and a translation parallel to the rotation axis. •As for simple rotations, only diad, triad, tetrad and hexad axes, that are consistent with Bravais lattice translation vectors can be used for a screw operator. •In addition, the translation on each rotation must be a rational fraction of the entire translation. •There is no combination of rotations or translations that can transform the pattern produced by 31 to the pattern of 32 , and 41 to the pattern of 43, etc. •Thus, the screw operation results in handedness Class21/2 or chirality (can’t superimpose image on another, e.g., mirror image) to the pattern. -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
I`mt`qx1/00Lhmdq`knesgdLnmsg9Rbnkdbhsd This month’s mineral, scolecite, is an uncommon zeolite from India. Our write-up explains its origin as a secondary mineral in volcanic host rocks, the difficulty of collecting this fragile mineral, the unusual properties of the zeolite-group minerals, and why mineralogists recently revised the system of zeolite classification and nomenclature. OVERVIEW PHYSICAL PROPERTIES Chemistry: Ca(Al2Si3O10)A3H2O Hydrous Calcium Aluminum Silicate (Hydrous Calcium Aluminosilicate), usually containing some potassium and sodium. Class: Silicates Subclass: Tectosilicates Group: Zeolites Crystal System: Monoclinic Crystal Habits: Usually as radiating sprays or clusters of thin, acicular crystals or Hairlike fibers; crystals are often flattened with tetragonal cross sections, lengthwise striations, and slanted terminations; also massive and fibrous. Twinning common. Color: Usually colorless, white, gray; rarely brown, pink, or yellow. Luster: Vitreous to silky Transparency: Transparent to translucent Streak: White Cleavage: Perfect in one direction Fracture: Uneven, brittle Hardness: 5.0-5.5 Specific Gravity: 2.16-2.40 (average 2.25) Figure 1. Scolecite. Luminescence: Often fluoresces yellow or brown in ultraviolet light. Refractive Index: 1.507-1.521 Distinctive Features and Tests: Best field-identification marks are acicular crystal habit; vitreous-to-silky luster; very low density; and association with other zeolite-group minerals, especially the closely- related minerals natrolite [Na2(Al2Si3O10)A2H2O] and mesolite [Na2Ca2(Al6Si9O30)A8H2O]. Laboratory tests are often needed to distinguish scolecite from other zeolite minerals. Dana Classification Number: 77.1.5.5 NAME The name “scolecite,” pronounced SKO-leh-site, is derived from the German Skolezit, which comes from the Greek sklx, meaning “worm,” an allusion to the tendency of its acicular crystals to curl when heated and dehydrated. -

Types of Lattices
Types of Lattices Types of Lattices Lattices are either: 1. Primitive (or Simple): one lattice point per unit cell. 2. Non-primitive, (or Multiple) e.g. double, triple, etc.: more than one lattice point per unit cell. Double r2 cell r1 r2 Triple r1 cell r2 r1 Primitive cell N + e 4 Ne = number of lattice points on cell edges (shared by 4 cells) •When repeated by successive translations e =edge reproduce periodic pattern. •Multiple cells are usually selected to make obvious the higher symmetry (usually rotational symmetry) that is possessed by the 1 lattice, which may not be immediately evident from primitive cell. Lattice Points- Review 2 Arrangement of Lattice Points 3 Arrangement of Lattice Points (continued) •These are known as the basis vectors, which we will come back to. •These are not translation vectors (R) since they have non- integer values. The complexity of the system depends upon the symmetry requirements (is it lost or maintained?) by applying the symmetry operations (rotation, reflection, inversion and translation). 4 The Five 2-D Bravais Lattices •From the previous definitions of the four 2-D and seven 3-D crystal systems, we know that there are four and seven primitive unit cells (with 1 lattice point/unit cell), respectively. •We can then ask: can we add additional lattice points to the primitive lattices (or nets), in such a way that we still have a lattice (net) belonging to the same crystal system (with symmetry requirements)? •First illustrate this for 2-D nets, where we know that the surroundings of each lattice point must be identical. -

Group Theory Applied to Crystallography
International Union of Crystallography Commission on Mathematical and Theoretical Crystallography Summer School on Mathematical and Theoretical Crystallography 27 April - 2 May 2008, Gargnano, Italy Group theory applied to crystallography Bernd Souvignier Institute for Mathematics, Astrophysics and Particle Physics Radboud University Nijmegen The Netherlands 29 April 2008 2 CONTENTS Contents 1 Introduction 3 2 Elements of space groups 5 2.1 Linearmappings .................................. 5 2.2 Affinemappings................................... 8 2.3 AffinegroupandEuclideangroup . .... 9 2.4 Matrixnotation .................................. 12 3 Analysis of space groups 14 3.1 Lattices ....................................... 14 3.2 Pointgroups..................................... 17 3.3 Transformationtoalatticebasis . ....... 19 3.4 Systemsofnonprimitivetranslations . ......... 22 4 Construction of space groups 25 4.1 Shiftoforigin................................... 25 4.2 Determining systems of nonprimitivetranslations . ............. 27 4.3 Normalizeraction................................ .. 31 5 Space group classification 35 5.1 Spacegrouptypes................................. 35 5.2 Arithmeticclasses............................... ... 36 5.3 Bravaisflocks.................................... 37 5.4 Geometricclasses................................ .. 39 5.5 Latticesystems .................................. 41 5.6 Crystalsystems .................................. 41 5.7 Crystalfamilies ................................. .. 42 6 Site-symmetry -

Molecular Symmetry
Molecular Symmetry Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) – used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. Symmetrical : implies the species possesses a number of indistinguishable configurations. 1 Group Theory : mathematical treatment of symmetry. symmetry operation – an operation performed on an object which leaves it in a configuration that is indistinguishable from, and superimposable on, the original configuration. symmetry elements – the points, lines, or planes to which a symmetry operation is carried out. Element Operation Symbol Identity Identity E Symmetry plane Reflection in the plane σ Inversion center Inversion of a point x,y,z to -x,-y,-z i Proper axis Rotation by (360/n)° Cn 1. Rotation by (360/n)° Improper axis S 2. Reflection in plane perpendicular to rotation axis n Proper axes of rotation (C n) Rotation with respect to a line (axis of rotation). •Cn is a rotation of (360/n)°. •C2 = 180° rotation, C 3 = 120° rotation, C 4 = 90° rotation, C 5 = 72° rotation, C 6 = 60° rotation… •Each rotation brings you to an indistinguishable state from the original. However, rotation by 90° about the same axis does not give back the identical molecule. XeF 4 is square planar. Therefore H 2O does NOT possess It has four different C 2 axes. a C 4 symmetry axis. A C 4 axis out of the page is called the principle axis because it has the largest n . By convention, the principle axis is in the z-direction 2 3 Reflection through a planes of symmetry (mirror plane) If reflection of all parts of a molecule through a plane produced an indistinguishable configuration, the symmetry element is called a mirror plane or plane of symmetry . -

The Cubic Groups
The Cubic Groups Baccalaureate Thesis in Electrical Engineering Author: Supervisor: Sana Zunic Dr. Wolfgang Herfort 0627758 Vienna University of Technology May 13, 2010 Contents 1 Concepts from Algebra 4 1.1 Groups . 4 1.2 Subgroups . 4 1.3 Actions . 5 2 Concepts from Crystallography 6 2.1 Space Groups and their Classification . 6 2.2 Motions in R3 ............................. 8 2.3 Cubic Lattices . 9 2.4 Space Groups with a Cubic Lattice . 10 3 The Octahedral Symmetry Groups 11 3.1 The Elements of O and Oh ..................... 11 3.2 A Presentation of Oh ......................... 14 3.3 The Subgroups of Oh ......................... 14 2 Abstract After introducing basics from (mathematical) crystallography we turn to the description of the octahedral symmetry groups { the symmetry group(s) of a cube. Preface The intention of this account is to provide a description of the octahedral sym- metry groups { symmetry group(s) of the cube. We first give the basic idea (without proofs) of mathematical crystallography, namely that the 219 space groups correspond to the 7 crystal systems. After this we come to describing cubic lattices { such ones that are built from \cubic cells". Finally, among the cubic lattices, we discuss briefly the ones on which O and Oh act. After this we provide lists of the elements and the subgroups of Oh. A presentation of Oh in terms of generators and relations { using the Dynkin diagram B3 is also given. It is our hope that this account is accessible to both { the mathematician and the engineer. The picture on the title page reflects Ha¨uy'sidea of crystal structure [4]. -
Solids, Nets, and Cross Sections
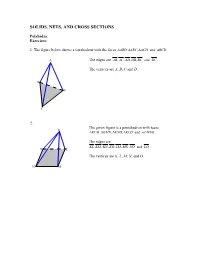
SOLIDS, NETS, AND CROSS SECTIONS Polyhedra: Exercises: 1. The figure below shows a tetrahedron with the faces ∆ABD,,∆∆ ABC ACD and ∆ BCD . A The edges are AB,,,, AC AD DB BC and DC . The vertices are A, B, C and D, D B C 2. The given figure is a pentahedron with faces K ∆KLM,∆∆∆ KMN , KNO , KLO and . LMNO . The edges are KL,,,,,, KM KN KO LM MN NO and LO . O L The vertices are K, L, M, N, and O. N M Prisms: Exercises (page-4): 1. The right regular octagonal prism has 8 lateral faces, so totally it has 10 faces. It has 24 edges, 8 of them are lateral edges. The prism has 16 vertices. 2. The figure below illustrates an oblique regular triangular prism. It has 5 faces (3 of them are lateral faces), 3 lateral edges and totally 9 edges. The prism has 6 vertices. Pyramids Exercises (page-6): 1. The pentagonal pyramid has 5 lateral faces and thus 6 faces in total. It has 5 lateral edges, 10 edges and 6 vertices. 2. The hexagonal pyramid has 6 lateral faces and 7 faces in total. It has 6 lateral edges, 12 edges and 7 vertices. Nets Exercises: 1. Regular right hexagonal pyramid 2. Rectangular prism 3. Regular right octagonal prism 4. Regular oblique hexagonal prism 5. and so on… 6. Right Cylinder 7. Right Cone Cross sections: Exercises (page-15): 1. i) In the cross section parallel to the base, the cross section is a regular hexagon of the same size as the base. -

Year 6 – Wednesday 24Th June 2020 – Maths
1 Year 6 – Wednesday 24th June 2020 – Maths Can I identify 3D shapes that have pairs of parallel or perpendicular edges? Parallel – edges that have the same distance continuously between them – parallel edges never meet. Perpendicular – when two edges or faces meet and create a 90o angle. 1 4. 7. 10. 2 5. 8. 11. 3. 6. 9. 12. C1 – Using the shapes above: 1. Name and sort the shapes into: a. Pyramids b. Prisms 2. Draw a table to identify how many faces, edges and vertices each shape has. 3. Write your own geometric definition: a. Prism b. pyramid 4. Which shape is the odd one out? Explain why. C2 – Using the shapes above; 1. Which of the shapes have pairs of parallel edges in: a. All their faces? b. More than one half of the faces? c. One face only? 2. The following shapes have pairs of perpendicular edges. Identify the faces they are in: 2 a. A cube b. A square based pyramid c. A triangular prism d. A cuboid 3. Which shape with straight edges has no perpendicular edges? 4. Which shape has perpendicular edges in the shape but not in any face? C3 – Using the above shapes: 1. How many faces have pairs of parallel edges in: a. A hexagonal pyramid? b. A decagonal (10-sided) based prism? c. A heptagonal based prism? d. Which shape has no face with parallel edges but has parallel edges in the shape? 2. How many faces have perpendicular edges in: a. A pentagonal pyramid b. A hexagonal pyramid c. -

Space Symmetry, Space Groups
Space symmetry, Space groups - Space groups are the product of possible combinations of symmetry operations including translations. - There exist 230 different space groups in 3-dimensional space - Comparing to point groups, space groups have 2 more symmetry operations (table 1). These operations include translations. Therefore they describe not only the lattice but also the crystal structure. Table 1: Additional symmetry operations in space symmetry, their description, symmetry elements and Hermann-Mauguin symbols. symmetry H-M description symmetry element operation symbol screw 1. rotation by 360°/N screw axis NM (Schraubung) 2. translation along the axis (Schraubenachse) 1. reflection across the plane glide glide plane 2. translation parallel to the a,b,c,n,d (Gleitspiegelung) (Gleitspiegelebene) glide plane Glide directions for the different gilde planes: a (b,c): translation along ½ a or ½ b or ½ c, respectively) (with ,, cba = vectors of the unit cell) n: translation e.g. along ½ (a + b) d: translation e.g. along ¼ (a + b), d from diamond, because this glide plane occurs in the diamond structure Screw axis example: 21 (N = 2, M = 1) 1) rotation by 180° (= 360°/2) 2) translation parallel to the axis by ½ unit (M/N) Only 21, (31, 32), (41, 43), 42, (61, 65) (62, 64), 63 screw axes exist in parenthesis: right, left hand screw axis Space group symbol: The space group symbol begins with a capital letter (P: primitive; A, B, C: base centred I, body centred R rhombohedral, F face centred), which represents the Bravais lattice type, followed by the short form of symmetry elements, as known from the point group symbols.