Mutagenic Analysis of Fermenting Strains and Fermented Brine for Stinky Tofu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study on Sodium Content in Local Foods
Annex I Annex I: Sodium content in non-prepackaged foods by category Food category (Food items included) n Sodium (mg/100g) Avg Std Dev Min Max Condiments and sauces 30 1,183 1,137 310 4,600 Sauce for Siumei/Lomei meat (Charsiew/ Siumei/ Lomei sauce; Ginger puree/ Ginger and shallot puree) 6 2,885 1,495 310 4,600 Curry gravy (Indian; Japanese; Thai)(Solid included) 6 635 135 390 790 White gravy (including mushroom; corn; etc.)(Solid included) 6 485 75 410 580 Asian sauces (Vietnamese sweet and sour sauce; Sauce for nuggets) 6 1,300 597 400 2,100 Gravy for other meat (Black pepper; Onion; Brown) 6 612 229 380 880 Processed meat products 80 1,225 1,250 280 6,800 Siumei/ Lomei chicken (Soy sauce chicken meat) 7 570 262 320 970 Siumei/ Lomei duck/ goose ("Lo shui" duck/goose; Roasted duck/goose) 9 738 347 360 1,400 Other siumei/ lomei poultry ("Lo shui" pigeon; Roasted pigeon) 7 669 301 280 1,000 Siumei/ Lomei pork (Roasted pork/ Roasted suckling pig; "Barbeque" pork) 9 691 193 350 970 Other siumei/ lomei pork (Salted and smoked pork; "Lo Shui" pork meat (ear; trotter; tongue)) 7 1,199 475 590 1,800 product Asian preserved sausages (Canton-style pork sausage/ Liver sausage; Red pork sausage) 5 1,754 775 870 2,700 Western preserved sausages (Meat; Cheese; Cervelat; Pork; Chicken) 4 933 70 840 1,000 Ready-to-eat marinated offal (Ox offals; Chicken liver) 4 585 283 330 990 Ready-to-eat meat balls (Fish ball (fried/boiled); Beef/ Beef tendon ball; Meat stuffed ball; Cuttle 10 744 205 420 980 fish ball; Shrimp ball) Preserved fish and seafood -

Soy Products As Healthy and Functional Foods
Middle-East Journal of Scientific Research 7 (1): 71-80, 2011 ISSN 1990-9233 © IDOSI Publications, 2011 Soy Products as Healthy and Functional Foods Hossein Jooyandeh Department of Food Science and Technology, Ramin Agricultural and Natural Resources University, Mollasani, Khuzestan, Iran Abstract: Over the recent decades, researchers have documented the health benefits of soy protein, especially for those who take soy protein daily. Soy products offer a considerable appeal for a growing segment of consumers with certain dietary and health concerns. It is quite evident that soy products do reduce the risks of developing various age-related chronic diseases and epidemiologic data strongly suggest that populations that regularly consume soy products have reduced incidence and prevalence of the aforementioned age-related conditions and diseases than populations that eat very little soy. The subject of what specific components is responsible for the plethora of reported health benefits of soybean remains a strong controversial issue, as the scientific community continues to understand what component(s) in soy is /are responsible for its health benefits. Soy constituents’ benefits mostly relate to the reduction of cholesterol levels and menopause symptoms and the reduction of the risk for several chronic diseases such as cancer, heart disease and osteoporosis. A variety of soy products are available on the market with different flavors and textures and a low-fat, nutritionally balanced diet can be developed from them. This article summarized the beneficial health, nutritional and functional properties of the soy ingredients and intends to illustrate the most current knowledge with a consciousness to motivate further research to optimize their favorable effects. -

Taipei Guide Taipei Guide Money
TAIPEI GUIDE TAIPEI GUIDE MONEY Currency: New Taiwanese Dollar (TWD/NT) = 100 Bottle of water at supermarket (1.5 liter) – 30 Essential Information cents. NT Money 3 Domestic beer (0.5 liter, draught) – 50 NT The easiest way to get Taiwanese money is to use Cappuccino – 80 NT Communication 4 This enchanting metropolis always seems to the ATMs that are literally on every corner or in Gasoline (1 liter) – 32 NT be busy, never slowing down or taking time to 7-Elevens (part of the international Plus or Cirrus Hostels (average price/night) – 400 NT Holidays 5 relax. Taipei has all the typical characteristics networks). You can also exchange money in the 4* hotel (average price/night) – 4000 NT banks (second most common option) or in hotels, Car-hire (medium-sized car/day) – 3000 NT Transportation 6 of a contemporary Asian global city: an excel- lent public transportation system, a glitzy cen- but the rates are not as good there. Private ex- Food 8 tral business district with signature skyscrap- change offices are not widespread in Taiwan. Tipping ers, overwhelming shopping malls and busy Tipping is not customary in Taiwan. The restau- Events During The Year 9 nightspots. Major cards (Visa, Master Card, JCB) are widely ac- rants usually include a service charge in the bill. Despite the erratic traffic, overcrowded streets cepted and paying with them is very convenient However, it is usual to tip bellhops in good hotels 10 Things to do and pollution, Taipei has also an amiable face in Taiwan. Amex and Diners club are accepted at (around 100NT) or staff in expat bars/clubs. -

Chinese Cuisine from Wikipedia, the Free Encyclopedia "Chinese Food
Chinese cuisine From Wikipedia, the free encyclopedia "Chinese food" redirects here. For Chinese food in America, see American Chinese cuisine. For other uses, see Chinese food (disambiguation). Chao fan or Chinese fried rice ChineseDishLogo.png This article is part of the series Chinese cuisine Regional cuisines[show] Overseas cuisine[show] Religious cuisines[show] Ingredients and types of food[show] Preparation and cooking[show] See also[show] Portal icon China portal v t e Part of a series on the Culture of China Red disc centered on a white rectangle History People Languages Traditions[show] Mythology and folklore[show] Cuisine Festivals Religion[show] Art[show] Literature[show] Music and performing arts[show] Media[show] Sport[show] Monuments[show] Symbols[show] Organisations[show] Portal icon China portal v t e Chinese cuisine includes styles originating from the diverse regions of China, as well as from Chinese people in other parts of the world including most Asia nations. The history of Chinese cuisine in China stretches back for thousands of years and has changed from period to period and in each region according to climate, imperial fashions, and local preferences. Over time, techniques and ingredients from the cuisines of other cultures were integrated into the cuisine of the Chinese people due both to imperial expansion and from the trade with nearby regions in pre-modern times, and from Europe and the New World in the modern period. In addition, dairy is rarely—if ever—used in any recipes in the style. The "Eight Culinary Cuisines" of China[1] are Anhui, Cantonese, Fujian, Hunan, Jiangsu, Shandong, Sichuan, and Zhejiang cuisines.[2] The staple foods of Chinese cooking include rice, noodles, vegetables, and sauces and seasonings. -

English Writing Unforgettable Flavor-Stinky Tofu:Why Does Stinky Tofu
English writing Unforgettable flavor -Stinky Tofu:Why does stinky tofu let the human love and hate? Author: Fang Yu-Chi( 方毓祺)。National Tainan Commercial Vocational Senior High School 。Apply Foreign Language 3B Syu Wun-Yi( 許文怡)。National Tainan Commercial Vocational Senior High School 。Apply Foreign Language 3B Liu Ya-Jhen( 劉雅禎)。National Tainan Commercial Vocational Senior High School 。 Apply Foreign Language 3B Instructor:Huang Yan-Hong( 黃燕鴻老師) Unforgettable flavor -Stinky Tofu:Why does stinky tofu let the human love and hate? I. INTRODUCTION A. Research Background Food is what matters to the people, and the good food is too numerous to mention in the world. Especially the Chinese traditional good food spreads most widely in this multiplex global village. The good food and the culture exchange mutually between country and country, and then become creative and changeable delicacies which are distinctive. The Chinese invented the food which raises the face to improve looks and nutrition -tofu. Tofu is one of the main foods in Vietnam, Thailand, South Korea and Japan and so on. Along with the East and West cultural exchange, people can buy tofu at some markets. Each region develops all kinds of different tofu ingredients such as Japanese soft tofu, Korean kimchi tofu, Guangdong deep-fried tofu, Chinese bean jelly and Taiwan deep-fried tofu. The fresh tofu through the fermentation, pickled, fried and many processes, and combination of sour-sweet and mouth-watering kimchi. These two food seem very ordinary, and do not connect, but they match actually make the human for it exclamation. After the elder evolves, it becomes the famous Chinese traditional snack nowadays -Stinky Tofu. -

Read Book the Food of Taiwan Ebook Free Download
THE FOOD OF TAIWAN PDF, EPUB, EBOOK Cathy Erway | 240 pages | 21 Apr 2015 | HOUGHTON MIFFLIN | 9780544303010 | English | Boston, United States The Food of Taiwan PDF Book Part travelogue and part cookbook, this book delves into the history of Taiwan and the author's own family history as well. French Food at Home. Categories: Side dish; Taiwanese; Vegan; Vegetarian Ingredients: light soy sauce; Chinese white rice wine; chayote shoots. Recently, deep-fried vegetarian rolls wrapped in tofu sheets have appeared in this section of the offering. Bowls of sweet or salty soy milk are classic Taiwanese breakfast fodder, accompanied by a feast of spongy, focaccia-like shao bing sesame sandwiches ; crispy dan bing egg crepes ; and long, golden- fried you tiao crullers. Your email address will not be published. Home 1 Books 2. The switch from real animals to noodles was made over a decade ago, we were told, to cut costs and reduce waste. For example, the San Bei Ji was so salty it was borderline inedible, while the Niu Rou Mian was far heavier on the soy sauce than any version I've had in Taiwan. Hardcover , pages. Little has changed over the years in terms of the nature of the ceremony and the kind of attire worn by the participants, but there have been some surprising innovations in terms of what foods are offered and how they are handled. Aside from one-off street stalls and full-blown restaurants, there are a few other unexpected spots for a great meal. I have to roll my eyes when she says that Taiwan is "diverse" even though it has a higher percentage of Han Chinese than mainland China does and is one of the most ethnically homogeneous states in the world. -

When Chinese Cuisine Meets Western Wine 9 Q1 Shu-Tai Wang 11 Department of Hospitality Management, Tunghai University, P.O
Prod:Type:FTP ED: 5þ model IJGFS : 52 pp:029ðcol:fig::NILÞ PAGN: SCAN: Available online at www.sciencedirect.com International Journal of 1 Gastronomy and Food Science 3 International Journal of Gastronomy and Food Science ] (]]]]) ]]]–]]] www.elsevier.com/locate/ijgfs 5 7 When Chinese cuisine meets western wine 9 Q1 Shu-Tai Wang 11 Department of Hospitality Management, Tunghai University, P.O. Box 891, Taichung 407, Taiwan 13 Received 7 March 2016; accepted 16 November 2016 15 17 Abstract 19 This study explores the guiding principle for pairing common western table wines such as Chardonnay, Riesling, Merlot and Cabernet Sauvignon with authentic Chinese cuisines. Sensory evaluation was carried out to measure the affective level on the pairing of food and wine. A fi 21 ve by eight (4 different wines and no wine pairing with 8 different cuisines) factorial experiment design was carried out to attain the sensory affection from the taste panel. Hedonic rating was adopted to assess the affective response of the cuisine and wine pairings. The results of affective test indicated that Riesling was the preferred wine to pair with most of the Chinese cuisines in question. The interaction was significant 23 between different cuisines and wines (p¼0.000), indicating that the hedonic sensory pattern of the cuisine can be influenced by the type of wine paired. In addition, Multidimensional Scaling (MDS) graph was proven to be an effective tool for visualizing the guiding principle of food and 25 wine pairing. & 2016 AZTI-Tecnalia. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license 27 (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

Acknowledgments
University of Groningen Controlled magnon spin transport in insulating magnets Liu, Jing DOI: 10.33612/diss.97448775 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2019 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Liu, J. (2019). Controlled magnon spin transport in insulating magnets: from linear to nonlinear regimes. University of Groningen. https://doi.org/10.33612/diss.97448775 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 26-09-2021 Acknowledgments Even though you’re alone in your boat, it’s always comforting to see the lights of the other boats bobbing nearby.* I would like to give my acknowledgement to those people and experiences which provided these lights during my time in Groningen. They are directly or indirectly related to the work presented in this thesis but are all deeply bonded to the accomplishment of this little booklet. -

Download Entire TAIPEI
STORIES FROM THE CAPITAL SUMMER 2019 VOL.16 MICHELIN X TAIPEI Michelin Guide Showcases Taipei’s Thriving Culinary Scene Three Alternative Ways to Experience Food in Taipei Traveling to Taipei During Ghost Month Dreaming of Food in Taipei YouTube Star Ku Shares His Love of Local Taiwanese Eats SUMMER 2019 VOL.16 TAIPEI IS AVAILABLE AT 臺北市政府觀光傳播局 南港軟體工業園區 臺北市孔廟 Department of Information and Tourism, Nangang Software Park Taipei Confucius Temple Taipei City Government 2F, 19-10, Sanchong Rd., Taipei City 275, Dalong St., Taipei City 4F, 1, City Hall Rd., Taipei City (02) 2655-3093 ext.124 (02) 2592-3924 1999 ext.7564 臺北美國學校 松山文創園區 臺灣桃園國際航空站一 Taipei American School Shongshan Cultural and Creative Park Tourist Service Center at Arrival Lobby, 800, Sec. 6, Zhongshan N. Rd., Taipei City 133, Guanfu S. Rd., Taipei City Taiwan Taoyuan International Airport (02) 2873-9900 (02) 2765-1388 Terminal I 9, Hangzhan S. Rd., Taoyuan City (03) 398-2194 國立中正紀念堂 華山 1914 文化創意產業園區 National Chiang Kai-shek Memorial Hall Huashan 1914 Creative Park 21, Zhongshan S. Rd., Taipei City 1, Sec.1, Bade Rd., Taipei City 臺灣桃園國際航空站二 (02) 2343-1100 (02) 2358-1914 Tourist Service Center at Arrival Lobby, Taiwan Taoyuan International Airport Terminal II 台北當代藝術館 國立臺灣博物館 9, Hangzhan S. Rd., Taoyuan City Museum of Contemporary Art (MOCA), Taipei National Taiwan Museum (03) 398-3341 39, Changan W. Rd., Taipei City 2, Xiangyang Rd., Taipei City (02) 2552-3720 (02) 2382-2566 美國在臺協會 市長官邸藝文沙龍 American Institute in Taiwan 臺北市旅遊服務中心 7, Ln. 134, Sec. 3, Xinyi Rd., Taipei City Mayor’s Residence Arts Salon Visitor Information Centers in Taipei (02) 2162-2000 46, Xuzhou Rd., Taipei City (02) 2396-9398 捷運沿線各站 遠企購物中心 台北國際藝術村 All Stations of MRT Lines Taipei Metro the Mall 203, Sec. -

Recipe Collections
Edward R. Hamilton Bookseller Company • Falls Village, Connecticut A special selection of Cooking Instruction – Recipe Collections – Low Fat & Healthy Cooking Slow Cooking – Grilling – Vegetarian Cooking – Ethnic Cooking – Regional & Exotic Cuisines Holidays & Entertaining – Cookies, Breads & Baking – Canning & Preserving – Wine Selection Notable Chefs & Restaurants – Bartending Guides and much more. June 28, 2019 6975267 1000 SAUCES, DIPS AND 7582633 THE QUINTESSENTIAL QUINOA DRESSINGS. By Nadia Arumugam. Provides the COOKBOOK. By Wendy Polisi. Discover new ways guidance, inspiration and recipes needed to lift to enjoy this South American staple with quinoa meals, desserts, snacks and more to new heights recipes for every occasion. Try Strawberry Spinach of deliciousness. Jazz up your meals with white Quinoa Salad, Quinoa Burgers, Almond Fudge sauces; brown stock-based sauces; pesto sauces; Quinoa Brownies, and more. Also gives alternatives creamy dips; fusion and Asian sauces; oil and for many recipes, covering the needs of vegan, vinegar dressings; salsas; and more. Color gluten-free, and sugar-free diners. Color photos. photos. 288 pages. Firefly. Pub. at $29.95 $5.95 221 pages. Skyhorse. Pub. at $17.95 $2.95 2878410 QUICK-FIX DINNERS. Ed. by the 6857833 100 RECIPES: The Absolute eds. of Southern Living. There’s something for Best Ways to Make the True Essentials. By everyone in this collection. Recipe flags show the eds. at America’s Test Kitchen. Organized busy cooks at a glance how long a dish takes from into three sections, each recipe is preceded by a start to finish. There are ideas for comfort foods thought provoking essay that positions the dish. pasta night, dinners, side dishes and desserts, You’ll find useful workday recipes like a killer proving that dinner made fast can be flavorful, tomato sauce; genius techniques for producing satisfying, and best of all, stress free. -
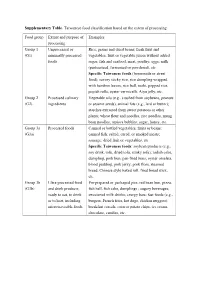
Supplementary Table. Taiwanese Food Classification Based on the Extent of Processing
Supplementary Table. Taiwanese food classification based on the extent of processing Food group Extent and purpose of Examples processing Group 1 Unprocessed or Rice, grains and dried beans; fresh fruit and (G1) minimally processed vegetables, fruit or vegetable juices without added foods sugar, fish and seafood, meat, poultry; eggs; milk (pasteurized, fermented or powdered), etc. Specific Taiwanese foods (homemade or street food): savory sticky rice, rice dumpling wrapped with bamboo leaves, rice ball, sushi, popped rice, popiah rolls, oyster vermicelli, Aiyu jelly, etc. Group 2 Processed culinary Vegetable oils (e.g., crushed from soybeans, peanuts (G2) ingredients or sesame seeds), animal fats (e.g., lard or butter); starches extracted from sweet potatoes or other plants; wheat flour and noodles, rice noodles, mung bean noodles, tapioca bubbles; sugar, honey, etc. Group 3a Processed foods Canned or bottled vegetables, fruits or beans; (G3a) canned fish; salted, cured, or smoked meats; sausage; dried fruit or vegetables, etc. Specific Taiwanese foods: soybean products (e.g., soy drink, tofu, dried tofu, stinky tofu); radish cake, dumpling, pork bun, pan-fried buns, oyster omelets, blood pudding, pork jerky, pork floss, steamed bread, Chinese style baked roll, fried bread stick, etc. Group 3b Ultra-processed food Pre-prepared or packaged pies, red bean bun, pizza, (G3b) and drink products; fish ball, fish cake, dumplings ; sugary beverages; ready to eat, to drink sweetened milk drinks; energy bars; fast foods (e.g., or to heat, including burgers, French fries, hot dogs, chicken nuggets); microwaveable foods breakfast cereals, corn or potato chips, ice cream, chocolate, candies, etc. . -

History of Fermented Black Soybeans 1
HISTORY OF FERMENTED BLACK SOYBEANS 1 HISTORY OF FERMENTED BLACK SOYBEANS (165 B.C. to 2011): EXTENSIVELY ANNOTATED BIBLIOGRAPHY AND SOURCEBOOK USED TO MAKE BLACK BEAN SAUCE. ALSO KNOW AS: FERMENTED BLACK BEANS, SALTED BLACK BEANS, FERMENTED SOYBEANS, PRESERVED BLACK BEANS, SALTY BLACK BEANS, BLACK FERMENTED BEANS, BLACK BEANS; DOUCHI, DOUSHI, TOUSHI, TOU-CH’IH, SHI, SHIH, DOW SEE, DOWSI (CHINESE); HAMANATTO, DAITOKUJI NATTO (JAPANESE); TAUSI, TAOSI (FILIPINO) Compiled by William Shurtleff & Akiko Aoyagi 2011 Copyright © 2011 by Soyinfo Center HISTORY OF FERMENTED BLACK SOYBEANS 2 Copyright (c) 2011 by William Shurtleff & Akiko Aoyagi All rights reserved. No part of this work may be reproduced or copied in any form or by any means - graphic, electronic, or mechanical, including photocopying, recording, taping, or information and retrieval systems - except for use in reviews, without written permission from the publisher. Published by: Soyinfo Center P.O. Box 234 Lafayette, CA 94549-0234 USA Phone: 925-283-2991 Fax: 925-283-9091 www.soyinfocenter.com [email protected] ISBN 978-1-928914-41-9 (Fermented Black Soybeans) Printed 11 Dec. 2011 Price: Available on the Web free of charge Search engine keywords: History of fermented black soybeans History of fermented black beans History of Hamanatto History of Hamananatto History of black bean sauce History of shi History of shih History of salted black beans History of fermented soybeans History of douchi History of doushi History of preserved soybeans History of dow see History of tausi