Informed Consent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2004 Albert Lasker Nomination Form
albert and mary lasker foundation 110 East 42nd Street Suite 1300 New York, ny 10017 November 3, 2003 tel 212 286-0222 fax 212 286-0924 Greetings: www.laskerfoundation.org james w. fordyce On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination Chairman neen hunt, ed.d. for the 2004 Albert Lasker Medical Research Awards. President mrs. anne b. fordyce The Awards will be offered in three categories: Basic Medical Research, Clinical Medical Vice President Research, and Special Achievement in Medical Science. This is the 59th year of these christopher w. brody Treasurer awards. Since the program was first established in 1944, 68 Lasker Laureates have later w. michael brown Secretary won Nobel Prizes. Additional information on previous Lasker Laureates can be found jordan u. gutterman, m.d. online at our web site http://www.laskerfoundation.org. Representative Albert Lasker Medical Research Awards Program Nominations that have been made in previous years may be updated and resubmitted in purnell w. choppin, m.d. accordance with the instructions on page 2 of this nomination booklet. daniel e. koshland, jr., ph.d. mrs. william mccormick blair, jr. the honorable mark o. hatfied Nominations should be received by the Foundation no later than February 2, 2004. Directors Emeritus A distinguished panel of jurors will select the scientists to be honored. The 2004 Albert Lasker Medical Research Awards will be presented at a luncheon ceremony given by the Foundation in New York City on Friday, October 1, 2004. Sincerely, Joseph L. Goldstein, M.D. Chairman, Awards Jury Albert Lasker Medical Research Awards ALBERT LASKER MEDICAL2004 RESEARCH AWARDS PURPOSE AND DESCRIPTION OF THE AWARDS The major purpose of these Awards is to recognize and honor individuals who have made signifi- cant contributions in basic or clinical research in diseases that are the main cause of death and disability. -

Research Organizations and Major Discoveries in Twentieth-Century Science: a Case Study of Excellence in Biomedical Research
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Hollingsworth, Joseph Rogers Working Paper Research organizations and major discoveries in twentieth-century science: A case study of excellence in biomedical research WZB Discussion Paper, No. P 02-003 Provided in Cooperation with: WZB Berlin Social Science Center Suggested Citation: Hollingsworth, Joseph Rogers (2002) : Research organizations and major discoveries in twentieth-century science: A case study of excellence in biomedical research, WZB Discussion Paper, No. P 02-003, Wissenschaftszentrum Berlin für Sozialforschung (WZB), Berlin This Version is available at: http://hdl.handle.net/10419/50229 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. www.econstor.eu P 02 – 003 RESEARCH ORGANIZATIONS AND MAJOR DISCOVERIES IN TWENTIETH-CENTURY SCIENCE: A CASE STUDY OF EXCELLENCE IN BIOMEDICAL RESEARCH J. -

Synthetic Biology in Fine Art Practice. Doctoral Thesis, Northumbria University
Citation: Mackenzie, Louise (2017) Evolution of the Subject – Synthetic Biology in Fine Art Practice. Doctoral thesis, Northumbria University. This version was downloaded from Northumbria Research Link: http://nrl.northumbria.ac.uk/38387/ Northumbria University has developed Northumbria Research Link (NRL) to enable users to access the University’s research output. Copyright © and moral rights for items on NRL are retained by the individual author(s) and/or other copyright owners. Single copies of full items can be reproduced, displayed or performed, and given to third parties in any format or medium for personal research or study, educational, or not-for-profit purposes without prior permission or charge, provided the authors, title and full bibliographic details are given, as well as a hyperlink and/or URL to the original metadata page. The content must not be changed in any way. Full items must not be sold commercially in any format or medium without formal permission of the copyright holder. The full policy is available online: http://nrl.northumbria.ac.uk/policies.html EVOLUTION OF THE SUBJECT SYNTHETIC BIOLOGY IN FINE ART PRACTICE LOUISE MACKENZIE PhD 2017 EVOLUTION OF THE SUBJECT SYNTHETIC BIOLOGY IN FINE ART PRACTICE LOUISE MACKENZIE A thesis submitted in partial fulfilment of the requirements of the University of Northumbria at Newcastle for the degree of Doctor of Philosophy Research undertaKen in the Faculty of Arts, Design & Social Sciences in collaboration with the Institute of Genetic Medicine at Newcastle University December 2017 ABSTRACT AcKnowledging a rise in the use of synthetic biology in art practice, this doctoral project draws from vital materialist discourse on biotechnology and biological materials in the worKs of Donna Haraway, Jane Bennett, Rosi Braidotti and Marietta Radomska to consider the liveliness of molecular biological material through art research and practice. -

Lasker Interactive Research Nom'18.Indd
THE 2018 LASKER MEDICAL RESEARCH AWARDS Nomination Packet albert and mary lasker foundation November 1, 2017 Greetings: On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination for the 2018 Lasker Medical Research Awards. Since 1945, the Lasker Awards have recognized the contributions of scientists, physicians, and public citizens who have made major advances in the understanding, diagnosis, treatment, cure, and prevention of disease. The Medical Research Awards will be offered in three categories in 2018: Basic Research, Clinical Research, and Special Achievement. The Lasker Foundation seeks nominations of outstanding scientists; nominations of women and minorities are encouraged. Nominations that have been made in previous years are not automatically reconsidered. Please see the Nomination Requirements section of this booklet for instructions on updating and resubmitting a nomination. The Foundation accepts electronic submissions. For information on submitting an electronic nomination, please visit www.laskerfoundation.org. Lasker Awards often presage future recognition of the Nobel committee, and they have become known popularly as “America’s Nobels.” Eighty-seven Lasker laureates have received the Nobel Prize, including 40 in the last three decades. Additional information on the Awards Program and on Lasker laureates can be found on our website, www.laskerfoundation.org. A distinguished panel of jurors will select the scientists to be honored with Lasker Medical Research Awards. The 2018 Awards will -

The Career of Maclyn Mccarty Joshua Lederberg*, Emil C
Open access, freely available online Obituary A Path to Discovery: The Career of Maclyn McCarty Joshua Lederberg*, Emil C. Gotschlich aclyn McCarty, who devoted his life as a physician-scientist Mto studying infectious disease organisms, was best known for his part in the monumental discovery that DNA, rather than protein, constituted the chemical nature of a gene. Uncovering the molecular secret of the gene in question—that for the capsular polysaccharide of pneumococcal bacteria—led the way to studying heredity not only through genetics but also through chemistry, and initiated the dawn of the age of molecular biology. McCarty was the youngest and longest surviving member of the research team responsible for this feat, which also included Oswald T. Avery and Colin MacLeod; he died on January 2, 2005, from congestive heart failure. McCarty was born in 1911 in South Bend, Indiana, the second of four sons of a branch manager for the Studebaker Corporation while it was DOI: 10.1371/journal.pbio.0030341.g001 still a fi rm for horse-drawn carriages. Maclyn McCarty (June 9, 1911, to January 2, 2005) with Francis Crick and James D. In his teens, McCarty set himself the Watson goal of becoming a physician-scientist, (Photo: Marjorie McCarty) and he pursued a successful strategy to prepare for admission to, and early pneumococcal transformation, the However, in 1928, Fred Griffi th, success in, Johns Hopkins University heritable alteration of a pneumococcal a leader in public-health research Medical School. As an undergraduate strain from a nonvirulent rough form in Britain, demonstrated that the at Stanford University, he presciently to a virulent smooth encapsulated conversion of one strain to another began his studies in the nascent fi eld form. -

Curriculum Vitae Helen Haskell Hobbs
CURRICULUM VITAE HELEN HASKELL HOBBS Birth Date; Place: May 5, 1952; Boston, Massachusetts Address: Department of Molecular Genetics University of Texas Southwestern Medical Center at Dallas 5323 Harry Hines Boulevard Dallas, Texas 75390-9046 Telephone: (214) 648-6724 Fax: (214) 648-7539 e-mail: [email protected] EDUCATION & TRAINING: 1970-1971 University of Pennsylvania, Philadelphia, PA 1971-1974 B.A., Human Biology, Stanford University, Palo Alto, CA 1975-1979 M.D., Case Western Reserve University School of Medicine, Cleveland, OH 1979-1980 Intern, Internal Medicine, Columbia Presbyterian Medical Center, New York City, NY 1980-1982 Resident, Internal Medicine, University of Texas Southwestern Medical Center (UT Southwestern), Dallas, TX 1982-1983 Chief Resident, Department of Internal Medicine, UT Southwestern 1983-1987 Postdoctoral Fellowship, Department of Molecular Genetics & Subspecialty Training in Endocrinology and Metabolism, UT Southwestern POSITIONS: 1987-1991 Assistant Professor, Departments of Internal Medicine and Molecular Genetics, and Chief, Division of Medical Genetics, UT Southwestern 1991-1995 Associate Professor, Departments of Internal Medicine and Molecular Genetics, and Chief, Division of Medical Genetics, UT Southwestern 1995- Professor, Departments of Internal Medicine and Molecular Genetics, and Chief, Division of Medical Genetics, UT Southwestern 2000- Director, McDermott Center for Human Growth and Development, UT Southwestern 2002- Investigator, Howard Hughes Medical Institute ELECTED MEMBERSHIPS: -

National Academy of Sciences July 1, 1973
NATIONAL ACADEMY OF SCIENCES JULY 1, 1973 OFFICERS Term expires President-PHILIP HANDLER June 30, 1975 Vice-President-SAUNDERS MAC LANE June 30, 1977 Home Secretary-ALLEN V. ASTIN June 30, 1975 Foreign Secretary-HARRISON BROWN June 30, 1974 Treasurer- E. R. PIORE June 30, 1976 Executive Officer Comptroller John S. Coleman Aaron Rosenthal Buiness Manager Bernard L. Kropp COUNCIL *Astin, Allen V. (1975) Marshak, Robert E. (1974) Babcock, Horace W. (1976) McCarty, Maclyn (1976) Bloch, Konrad E. (1974) Pierce, John R. (1974) Branscomb, Lewis M. (1975) *Piore, E. R. (1976) *Brown, Harrison (1974) Pitzer, Kenneth S. (1976) *Cloud, Preston (1975) *Shull, Harrison (1974) Eagle, Harry (1975) Westheimer, Frank H. (1975) *Handler, Philip (1975) Williams, Carroll M. (1976) *Mac Lane, Saunders (1977) * Members of the Executive Committee of the Council of the Academy. SECTIONS The Academy is divided into the following Sections, to which members are assigned at their own choice: (1) Mathematics (10) Microbiology (2) Astronomy (11) Anthropology (3) Physics (12) Psychology (4) Engineering (13) Geophysics (5) Chemistry (14) Biochemistry (6) Geology (15) Applied Biology (7) Botany (16) Applied Physical and Mathematical Sciences (8) Zoology (17) Medical Sciences (9) Physiology (18) Genetics (19) Social, Economic, and Political Sciences In the alphabetical list of members, the number in parentheses, following year of election, indicates the Section to WCiph the member belongs. 3009 Downloaded by guest on September 25, 2021 3010 Members N.A.S. Organization CLASSES Ames, Bruce Nathan, 1972 (14), Department of Biochem- istry, University of California, Berkeley, California The members of Sections are grouped in the following Classes: 94720 Anderson, Carl David, 1938 (3), California Institute of I. -
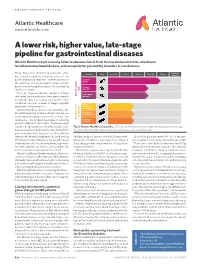
A Lower Risk, Higher Value, Late-Stage Pipeline for Gastrointestinal Diseases
ADVERTISEMENT FEATURE Atlantic Healthcare www.atlantichc.com A lower risk, higher value, late-stage pipeline for gastrointestinal diseases Atlantic Healthcare plc is raising funds to advance clinical trials for new chemical entities: alicaforsen for inflammatory bowel disease, and renzapride for gut motility disorders in rare diseases. Regulatory Many drugs miss clinical trial endpoints, often Indication Form Pre-clinical Phase 1 Phase 2a Phase 2b Phase 3 due to incorrect patient selection, choice of end approval points and dosing regimens. Further analysis of UC enema the data may reveal new insights and provide the Camligo basis for continuing development incorporating UC tablet AHC-1145 the lessons learnt. IBD There are many well-known examples of drugs Crohn’s tablet that failed for the indication they were originally Alicaforsen for AHC-1062 intended for, but successfully developed for other conditions; everyone is aware of Viagra, originally Systemic developed for hypertension. scleroderma GI complications Atlantic Healthcare, based near Cambridge, UK, Cystic fibrosis has established itself as a trans-Atlantic pharmaceu- GI complications tical company, building a diversified late-stage clini- GI Motility cal pipeline. The company specializes in acquiring Renzapride for Undisclosed indication products with robust clinical data. “We have brought together deep expertise to identify, acquire, com- Fig. 1 | Atlantic Healthcare’s pipeline. Offering risk diversification and targeting multiple patient groups. plete development, and market products directly to GI, gastrointestinal; IBD, inflammatory bowel disease; UC, ulcerative colitis. gastrointestinal (GI) clinicians,” said Toby Wilson Waterworth, Atlantic Healthcare’s CEO, and a Fellow William Sandborn, Director of the IBD Center at the GI motility disorders affect 10–15% of the gen- of the Royal Society of Medicine. -

Discovering Genes Are Made of DNA Maclyn Mccarty
feature Discovering genes are made of DNA Maclyn McCarty The Rockefeller University, 1230 York Avenue, New York 10021, USA (e-mail: [email protected]) Maclyn McCarty is the sole surviving member of the team that made the remarkable discovery that DNA is the material of inheritance. This preceded by a decade the discovery of the structure of DNA itself. Here he shares his personal perspective of those times and the impact of the double helix. Editor’s note — For a long time, biologists thought that ‘genes’, the units of inheritance, were made up of protein. In 1944, in what was arguably the defining moment for nucleic acid research, Oswald Avery, Maclyn McCarty and Colin MacLeod, at Rockefeller Institute (now University) Hospital, New York, proved that DNA was the material of inheritance, the so-called stuff of life. They showed that the heritable property of virulence from one infectious strain of pneumococcus (the bacterial agent of pneumonia) could be transferred to a noninfectious bacterium with pure DNA1. They further supported their conclusions by showing that this ‘transforming’ activity could be destroyed by the DNA-digesting enzyme DNAase2,3. This work first linked genetic information with DNA and provided the historical platform of modern genetics. Their discovery was greeted initially with scepticism, however, in part because many scientists believed that DNA was too simple a molecule to be the genetic material. And the fact that McCarty, Avery and MacLeod were not awarded the Nobel prize is an oversight that, to this day, still puzzles. “The pivotal t the time of our discovery and publication isolated from various sources, and that despite this discovery of in 1944 (ref. -

Alicaforsen, an Antisense Inhibitor of ICAM-1, As Treatment for Left- Sided Ulcerative Colitis and Ulcerative Proctitis
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2017 Alicaforsen in the treatment of pouchitis Greuter, Thomas ; Rogler, Gerhard Abstract: Alicaforsen is a 20-base antisense oligonucleotide inhibiting ICAM-1 production, which is an important adhesion molecule involved in leukocyte migration and trafficking to the site of inflamma- tion. Hitherto, alicaforsen has been granted orphan drug designation and is prescribed as an unlicensed medicine in accordance with international regulation for the treatment of pouchitis and left-sided ulcer- ative colitis. Given the positive results evolving from one open-label trial and one case series in patients with chronic refractory pouchitis, US FDA has agreed to a rolling submission for a license application for the treatment of pouchitis, which has been recently initiated. Whether alicaforsen leads to higher endoscopic and clinical remission rates as placebo and whether the response can be maintained in the long-term in larger studies is yet unknown. An ongoing multicenter international Phase III trial will definitely address these unanswered questions. DOI: https://doi.org/10.2217/imt-2017-0085 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-145563 Journal Article Accepted Version Originally published at: Greuter, Thomas; Rogler, Gerhard (2017). Alicaforsen in the treatment of pouchitis. Immunotherapy, 9(14):1143-1152. DOI: https://doi.org/10.2217/imt-2017-0085 -

The Helen Hay Whitney Foundation Final - Year Fellows Fifty-Fifth Annual Fellows Meeting - November 2-4, 2012
THE HELEN HAY WHITNEY FOUNDATION 2012-2013 Annual REport 20 Squadron Boulevard, Suite 630 New City, NY 10956 www.hhwf.org Tel: (845) 639-6799 Fax: (845) 639-6798 THE HELEN HAY WHITNEY FOUNDATION BOARD OF TRUSTEES Averil Payson Meyer, President Steven C. Harrison, Ph.D., Vice President Lisa A. Steiner, M.D., Vice President W. Perry Welch, Treasurer Thomas M. Jessell, Ph.D. Payne W. Middleton Thomas P. Sakmar, M.D. Stephen C. Sherrill Jerome Gross, M.D., Trustee Emeritus SCIENTIFIC ADVISORY COMMITTEE Steven C. Harrison, Ph.D., Chairman David J. Anderson, Ph.D. Daniel Kahne, Ph.D. Philippa Marrack, Ph.D. Markus Meister, Ph.D. Barbara J. Meyer, Ph.D. Matthew D. Scharff, M.D. Julie A. Theriot, Ph.D. Jonathan S. Weissman, Ph.D. S. Lawrence Zipursky, Ph.D. ADMINISTRATIVE DIRECTOR and SECRETARY Robert Weinberger Page 1 of 29 REPORT OF THE VICE PRESIDENT AND CHAIRMAN, SCIENTIFIC ADVISORY COMMITTEE The past two years have been eventful ones. The generosity of the Simons Foundation has allowed us to increase by three the number of fellows we select each year, giving us a class of about 24. Tom Jessell, a former SAC member and now a Trustee, was instrumental in helping us approach the Simons Foundation, which has an extremely interesting overall plan for support of frontier biomedical research and with which I can foresee more extensive interactions going forward. We also increased by one the number of SAC members, to enhance coverage in fields such as systems neuroscience and mathematical modeling, in which we have received applications that have challenged the expertise of the eight-member group. -

LAY SUMMARY CLINICAL RESEARCH PROTOCOL ACH UCP-301 Eudract Number 2013-002952-34 IND Number 64,905
LAY SUMMARY CLINICAL RESEARCH PROTOCOL ACH UCP-301 EudraCT Number 2013-002952-34 IND Number 64,905 Who Sponsored the Study? Atlantic Pharmaceuticals Ltd, Atlantic House, 10 Rose & Crown Walk, Saffron Walden, Essex, CB10 1JH, United Kingdom. Phone: +44 (0)1799 619 413 Email: [email protected] Website: www.atlantic.com Title of this Phase 3 Study: A Randomised, Double-Blind, Placebo-Controlled Trial of the Safety and Efficacy of Topical Alicaforsen Enema in Subjects with Active, Chronic, Antibiotic Refractory Primary Idiopathic Pouchitis. - In a Phase 3 study a new treatment is tested in many patients. - In this study, researchers compared the test medicine (alicaforsen enema) to placebo (identical looking enema liquid but with no medicine in it). - Randomising patients, by putting people (by chance) into 2 equal sized treatment groups, helps to reduce the differences between the groups, making the comparison between the groups fairer. - This trial was also “double-blinded”. This means that neither patients nor doctors knew who was given which treatment. This was done to make sure that the trial results were not influenced in any way. There was also an optional open-label-access follow-on-phase available to patients in this study (except for patients in France, for whom the national regulatory agency declined this part of the study). Alicaforsen enema is an investigational product designed to work in inflammatory bowel disease. In this study, the target was pouchitis. As the sponsor for this study, we at Atlantic Pharmaceuticals Ltd feel it is important for you to know the results. When the study ended, we created a scientific report of all the data.