An Overview of Genetic Engineering
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA
GENES AND PROTEINS THAT CONTROL THE SECRETORY PATHWAY Nobel Lecture, 7 December 2013 by RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA. Introduction George Palade shared the 1974 Nobel Prize with Albert Claude and Christian de Duve for their pioneering work in the characterization of organelles interrelated by the process of secretion in mammalian cells and tissues. These three scholars established the modern field of cell biology and the tools of cell fractionation and thin section transmission electron microscopy. It was Palade’s genius in particular that revealed the organization of the secretory pathway. He discovered the ribosome and showed that it was poised on the surface of the endoplasmic reticulum (ER) where it engaged in the vectorial translocation of newly synthesized secretory polypeptides (1). And in a most elegant and technically challenging investigation, his group employed radioactive amino acids in a pulse-chase regimen to show by autoradiograpic exposure of thin sections on a photographic emulsion that secretory proteins progress in sequence from the ER through the Golgi apparatus into secretory granules, which then discharge their cargo by membrane fusion at the cell surface (1). He documented the role of vesicles as carriers of cargo between compartments and he formulated the hypothesis that membranes template their own production rather than form by a process of de novo biogenesis (1). As a university student I was ignorant of the important developments in cell biology; however, I learned of Palade’s work during my first year of graduate school in the Stanford biochemistry department. -

Unrestricted Immigration and the Foreign Dominance Of
Unrestricted Immigration and the Foreign Dominance of United States Nobel Prize Winners in Science: Irrefutable Data and Exemplary Family Narratives—Backup Data and Information Andrew A. Beveridge, Queens and Graduate Center CUNY and Social Explorer, Inc. Lynn Caporale, Strategic Scientific Advisor and Author The following slides were presented at the recent meeting of the American Association for the Advancement of Science. This project and paper is an outgrowth of that session, and will combine qualitative data on Nobel Prize Winners family histories along with analyses of the pattern of Nobel Winners. The first set of slides show some of the patterns so far found, and will be augmented for the formal paper. The second set of slides shows some examples of the Nobel families. The authors a developing a systematic data base of Nobel Winners (mainly US), their careers and their family histories. This turned out to be much more challenging than expected, since many winners do not emphasize their family origins in their own biographies or autobiographies or other commentary. Dr. Caporale has reached out to some laureates or their families to elicit that information. We plan to systematically compare the laureates to the population in the US at large, including immigrants and non‐immigrants at various periods. Outline of Presentation • A preliminary examination of the 609 Nobel Prize Winners, 291 of whom were at an American Institution when they received the Nobel in physics, chemistry or physiology and medicine • Will look at patterns of -

MED C-LIVE Transcript
JUNE 2020 Mario Capecchi, Ph.D. NOT FOR PUBLIC DISTRIBUTION Michael Keel My name is Michael Keel from New Jersey. And it is my honor to introduce Dr. Mario Capecchi. Dr. Capecchi is the Distinguished Professor of Human Genetics at the University of Utah School of Medicine. He is best known for his groundbreaking work in gene targeting, in mass embryo derived stem cells. He was awarded the Nobel Prize in Physiology for Medicine, for his work in finding ways to manipulate the mammalian genome by changing mammals’ genes. His research interests include analysis of neural development in mammals, gene therapy, and production of murine models of human genetic diseases, from cancer to neuropsychiatric disorders. Dr. Capecchi, welcome to the Congress of Future Medical Leaders. Dr. Capecchi Thank you. First of all, welcome to the Medical Leaders’ Congress. It's a pleasure to be here. My name is Mario. I'm going to be talking about gene targeting. Next slide please. My laboratory has developed a technology called gene targeting that allows you to change any gene in any conceivable manner in a living creature, such as a mouse. Next slide. Why do we do gene targeting? Next slide. There are many reasons, but they boil down to two. One is basic research and the other is applied research. In basic research, you investigate the biology of an animal. For example, how do you make a limb or a heart or a brain? Those are basic research questions. The other is applied research. That is we can use gene targeting to generate mice with human diseases. -

Introduction and Historical Perspective
Chapter 1 Introduction and Historical Perspective “ Nothing in biology makes sense except in the light of evolution. ” modified by the developmental history of the organism, Theodosius Dobzhansky its physiology – from cellular to systems levels – and by the social and physical environment. Finally, behaviors are shaped through evolutionary forces of natural selection OVERVIEW that optimize survival and reproduction ( Figure 1.1 ). Truly, the study of behavior provides us with a window through Behavioral genetics aims to understand the genetic which we can view much of biology. mechanisms that enable the nervous system to direct Understanding behaviors requires a multidisciplinary appropriate interactions between organisms and their perspective, with regulation of gene expression at its core. social and physical environments. Early scientific The emerging field of behavioral genetics is still taking explorations of animal behavior defined the fields shape and its boundaries are still being defined. Behavioral of experimental psychology and classical ethology. genetics has evolved through the merger of experimental Behavioral genetics has emerged as an interdisciplin- psychology and classical ethology with evolutionary biol- ary science at the interface of experimental psychology, ogy and genetics, and also incorporates aspects of neuro- classical ethology, genetics, and neuroscience. This science ( Figure 1.2 ). To gain a perspective on the current chapter provides a brief overview of the emergence of definition of this field, it is helpful -

A Short History of DNA Technology 1865 - Gregor Mendel the Father of Genetics
A Short History of DNA Technology 1865 - Gregor Mendel The Father of Genetics The Augustinian monastery in old Brno, Moravia 1865 - Gregor Mendel • Law of Segregation • Law of Independent Assortment • Law of Dominance 1865 1915 - T.H. Morgan Genetics of Drosophila • Short generation time • Easy to maintain • Only 4 pairs of chromosomes 1865 1915 - T.H. Morgan •Genes located on chromosomes •Sex-linked inheritance wild type mutant •Gene linkage 0 •Recombination long aristae short aristae •Genetic mapping gray black body 48.5 body (cross-over maps) 57.5 red eyes cinnabar eyes 67.0 normal wings vestigial wings 104.5 red eyes brown eyes 1865 1928 - Frederick Griffith “Rough” colonies “Smooth” colonies Transformation of Streptococcus pneumoniae Living Living Heat killed Heat killed S cells mixed S cells R cells S cells with living R cells capsule Living S cells in blood Bacterial sample from dead mouse Strain Injection Results 1865 Beadle & Tatum - 1941 One Gene - One Enzyme Hypothesis Neurospora crassa Ascus Ascospores placed X-rays Fruiting on complete body medium All grow Minimal + amino acids No growth Minimal Minimal + vitamins in mutants Fragments placed on minimal medium Minimal plus: Mutant deficient in enzyme that synthesizes arginine Cys Glu Arg Lys His 1865 Beadle & Tatum - 1941 Gene A Gene B Gene C Minimal Medium + Citruline + Arginine + Ornithine Wild type PrecursorEnz A OrnithineEnz B CitrulineEnz C Arginine Metabolic block Class I Precursor OrnithineEnz B CitrulineEnz C Arginine Mutants Class II Mutants PrecursorEnz A Ornithine -

2004 Albert Lasker Nomination Form
albert and mary lasker foundation 110 East 42nd Street Suite 1300 New York, ny 10017 November 3, 2003 tel 212 286-0222 fax 212 286-0924 Greetings: www.laskerfoundation.org james w. fordyce On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination Chairman neen hunt, ed.d. for the 2004 Albert Lasker Medical Research Awards. President mrs. anne b. fordyce The Awards will be offered in three categories: Basic Medical Research, Clinical Medical Vice President Research, and Special Achievement in Medical Science. This is the 59th year of these christopher w. brody Treasurer awards. Since the program was first established in 1944, 68 Lasker Laureates have later w. michael brown Secretary won Nobel Prizes. Additional information on previous Lasker Laureates can be found jordan u. gutterman, m.d. online at our web site http://www.laskerfoundation.org. Representative Albert Lasker Medical Research Awards Program Nominations that have been made in previous years may be updated and resubmitted in purnell w. choppin, m.d. accordance with the instructions on page 2 of this nomination booklet. daniel e. koshland, jr., ph.d. mrs. william mccormick blair, jr. the honorable mark o. hatfied Nominations should be received by the Foundation no later than February 2, 2004. Directors Emeritus A distinguished panel of jurors will select the scientists to be honored. The 2004 Albert Lasker Medical Research Awards will be presented at a luncheon ceremony given by the Foundation in New York City on Friday, October 1, 2004. Sincerely, Joseph L. Goldstein, M.D. Chairman, Awards Jury Albert Lasker Medical Research Awards ALBERT LASKER MEDICAL2004 RESEARCH AWARDS PURPOSE AND DESCRIPTION OF THE AWARDS The major purpose of these Awards is to recognize and honor individuals who have made signifi- cant contributions in basic or clinical research in diseases that are the main cause of death and disability. -

Eighty Years of Fighting Against Cancer in Serbia Ovarian Cancer Vaccine
News Eighty years of fighting against cancer Recent study by Kunle Odunsi et al., Roswell Park Cancer Institute, Buffalo, in Serbia New York, USA, showed an effects of vaccine based on NY-ESO-1, a „cancer This year on December 10th, Serbian society for the fight against cancer has testis“ antigen in preventing the recurrence of ovarian cancer. NY-ESO-1 is a ovarian celebrated the great jubilee - 80 years of its foundation. The celebration took „cancer-testis“ antigen expressed in epithelial cancer (EOC) and is place in Crystal Room of Belgrade’s Hyatt hotel, gathering many distinguished among the most immunogenic tumor antigens defined to date. presence + guests from the country and abroad. This remarkable event has been held Author’s previous study point to the role of of intraepithelial CD8 - under the auspices of the President of Republic of Serbia, Mr. Boris Tadić. infiltrating T lymphocytes in tumors that was associated with improved survival of The whole ceremony was presided by Prof. Dr. Slobodan Čikarić, actual presi- patients with the disease. The NY-ESO-1 peptide epitope, ESO157–170, is recog- + + dent of Society, who greeted guests and wished them a warm welcome. Than nized by HLA-DP4-restricted CD4 T cells and HLA-A2- and A24-restricted CD8 + followed the speech of Serbian health minister, Prof. Dr. Tomica Milosavljević T cells. To test whether providing cognate helper CD4 T cells would enhance who pointed out the importance of preventive measures and public education the antitumor immune response, Odunsi et al., conducted a phase I clinical trial along with timely diagnosis and multidisciplinary treatment in global fight of immunization with ESO157–170 mixed with incomplete Freund's adjuvant + against cancer in Serbia. -

DNA: the Timeline and Evidence of Discovery
1/19/2017 DNA: The Timeline and Evidence of Discovery Interactive Click and Learn (Ann Brokaw Rocky River High School) Introduction For almost a century, many scientists paved the way to the ultimate discovery of DNA and its double helix structure. Without the work of these pioneering scientists, Watson and Crick may never have made their ground-breaking double helix model, published in 1953. The knowledge of how genetic material is stored and copied in this molecule gave rise to a new way of looking at and manipulating biological processes, called molecular biology. The breakthrough changed the face of biology and our lives forever. Watch The Double Helix short film (approximately 15 minutes) – hyperlinked here. 1 1/19/2017 1865 The Garden Pea 1865 The Garden Pea In 1865, Gregor Mendel established the foundation of genetics by unraveling the basic principles of heredity, though his work would not be recognized as “revolutionary” until after his death. By studying the common garden pea plant, Mendel demonstrated the inheritance of “discrete units” and introduced the idea that the inheritance of these units from generation to generation follows particular patterns. These patterns are now referred to as the “Laws of Mendelian Inheritance.” 2 1/19/2017 1869 The Isolation of “Nuclein” 1869 Isolated Nuclein Friedrich Miescher, a Swiss researcher, noticed an unknown precipitate in his work with white blood cells. Upon isolating the material, he noted that it resisted protein-digesting enzymes. Why is it important that the material was not digested by the enzymes? Further work led him to the discovery that the substance contained carbon, hydrogen, nitrogen and large amounts of phosphorus with no sulfur. -

I. Hox Genes 2
School ofMedicine Oregon Health Sciences University CERTIFICATE OF APPROVAL This is certify that the Ph.D. thesis of WendyKnosp has been approved Mentor/ Advisor ~ Member Member QUANTITATIVE ANALYSIS OF HOXA13 FUNCTION IN THE DEVELOPING LIMB By Wendy M. lt<nosp A DISSERTATION Presented to the Department of Molecular and Medical Genetics and the Oregon Health & Science University School of Medicine in partial fulfillment of the requirements for the degree of Doctor of Philosophy August 2006 TABLE OF CONTENTS LIST OF FIGURES iv LIST OF ABBREVIATIONS vii ACKNOWLEDGEMENTS X ABSTRACT xii CHAPTER 1: Introduction 1 I. Hox genes 2 A. Discovery of Hox genes in Drosophila melanogaster 2 B. Hox cluster colinearity and conservation 7 C. Human Hox mutations 9 D. Hoxa13: HFGS and Guttmacher syndromes 10 II. The Homeodomain 12 A. Homeodomain structure 12 B. DNA binding 14 Ill. Limb development 16 A. Patterning of the limb axes 16 B. Digit formation 20 C. lnterdigital programmed cell death 21 IV. BMPs and limb development 23 A. BMP signaling in the limb 23 B. BMP target genes 27 V. Hoxa13 and embryonic development 30 A. The Hoxa13-GFP mouse model 30 B. Hoxa13 mutant phenotypes 34 C. HOXA 13 homeodomain 35 D. HOXA 13 protein-protein interactions 36 E. HOXA 13 target genes 37 VI. Hypothesis and Rationale 39 CHAPTER 2: HOXA13 regulates Bmp2 and Bmp7 40 I. Abstract 42 II. Introduction 43 Ill. Results 46 IV. Discussion 69 v. Materials and Methods 75 VI. Acknowledgements 83 11 CHAPTER 3: Quantitative analysis of HOXA13 function 84 HOXA 13 regulation of Sostdc1 I. -

Jewels in the Crown
Jewels in the crown CSHL’s 8 Nobel laureates Eight scientists who have worked at Cold Max Delbrück and Salvador Luria Spring Harbor Laboratory over its first 125 years have earned the ultimate Beginning in 1941, two scientists, both refugees of European honor, the Nobel Prize for Physiology fascism, began spending their summers doing research at Cold or Medicine. Some have been full- Spring Harbor. In this idyllic setting, the pair—who had full-time time faculty members; others came appointments elsewhere—explored the deep mystery of genetics to the Lab to do summer research by exploiting the simplicity of tiny viruses called bacteriophages, or a postdoctoral fellowship. Two, or phages, which infect bacteria. Max Delbrück and Salvador who performed experiments at Luria, original protagonists in what came to be called the Phage the Lab as part of the historic Group, were at the center of a movement whose members made Phage Group, later served as seminal discoveries that launched the revolutionary field of mo- Directors. lecular genetics. Their distinctive math- and physics-oriented ap- Peter Tarr proach to biology, partly a reflection of Delbrück’s physics train- ing, was propagated far and wide via the famous Phage Course that Delbrück first taught in 1945. The famous Luria-Delbrück experiment of 1943 showed that genetic mutations occur ran- domly in bacteria, not necessarily in response to selection. The pair also showed that resistance was a heritable trait in the tiny organisms. Delbrück and Luria, along with Alfred Hershey, were awarded a Nobel Prize in 1969 “for their discoveries concerning the replication mechanism and the genetic structure of viruses.” Barbara McClintock Alfred Hershey Today we know that “jumping genes”—transposable elements (TEs)—are littered everywhere, like so much Alfred Hershey first came to Cold Spring Harbor to participate in Phage Group wreckage, in the chromosomes of every organism. -

Genes, Genomes and Genetic Analysis
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION UNIT 1 © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORDefining SALE OR DISTRIBUTION and WorkingNOT FOR SALE OR DISTRIBUTION with Genes © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Chapter 1 Genes, Genomes, and Genetic Analysis Chapter 2 DNA Structure and Genetic Variation © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Molekuul/Science Photo Library/Getty Images. © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 9781284136609_CH01_Hartl.indd 1 08/11/17 8:50 am © Jones & Bartlett Learning, LLC -
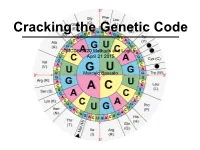
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J.