The Silent Period Induced by Transcranial Cranial Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
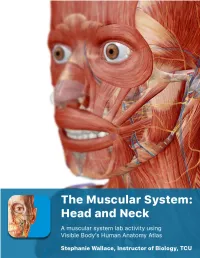
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

The Articulatory System Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D
The articulatory system Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D. STRUCTURE/FUNCTION VOCAL TRACT CLASSIFICATION OF CONSONANTS AND VOWELS MORE ON RESONANCE ACOUSTIC ANALYSIS/ SPECTROGRAMS SUPRSEGMENTALS, COARTICULATION 1 Midsagittal dissection From Kent, 1997 2 Oral Cavity 3 Oral Structures – continued • Moistened by saliva • Lined by mucosa • Saliva affected by meds 4 Tonsils • PALATINE* (laterally – seen in oral periph • LINGUAL (inf.- root of tongue) • ADENOIDS (sup.) [= pharyngeal] • Palatine, lingual tonsils are larger in children • *removed in tonsillectomy 5 Adenoid Facies • Enlargement from infection may cause problems (adenoid facies) • Can cause problems with nasal sounds or voicing • Adenoidectomy; also tonsillectomy (for palatine tonsils) 6 Adenoid faces (example) 7 Oral structures - frenulum Important component of oral periphery exam Lingual frenomy – for ankyloglossia “tongue-tie” Some doctors will snip for infants, but often will loosen by itself 8 Hard Palate Much variability in palate shape and height Very high vault 9 Teeth 10 Dentition - details Primary (deciduous, milk teeth) Secondary (permanent) n=20: n=32: ◦ 2 incisor ◦ 4 incisor ◦ 1 canine ◦ 2 canine ◦ 2 molar ◦ 4 premolar (bicuspid) Just for “fun” – baby ◦ 6 molar teeth pushing in! NOTE: x 2 for upper and lower 11 Types of malocclusion • Angle’s classification: • I, II, III • Also, individual teeth can be misaligned (e.g. labioversion) Also “Neutrocclusion/ distocclusion/mesiocclusion” 12 Dental Occlusion –continued Other terminology 13 Mandible Action • Primary movements are elevation and depression • Also…. protrusion/retraction • Lateral grinding motion 14 Muscles of Jaw Elevation Like alligators, we are much stronger at jaw elevation (closing to head) than depression 15 Jaw Muscles ELEVATORS DEPRESSORS •Temporalis ✓ •Mylohyoid ✓ •Masseter ✓ •Geniohyoid✓ •Internal (medial) Pterygoid ✓ •Anterior belly of the digastric (- Kent) •Masseter and IP part of “mandibular sling” •External (lateral) pterygoid(?)-- also protrudes and rocks side to side. -

Dr. Maue-Dickson Is Associate Professor of Pediat- Rics, University of Miami, Mailman Center for Child Development, University
Section II. Anatomy and Physiology WILMA MAUE-DICKSON, Ph.D. (CHAIRMAN) Introduction Middle Ear Musculature, The Auditory Tube, and The Velopharyngeal This Section has been prepared for the Mechanism purpose of updating the previous report, "Status of Research in Cleft Palate: Anat- 1. Tur Mippour® Ear omy and Physiology," published in two parts in the Cleft Palate Journal, Volume 11, The authors of the previous report 1974, and Volume 12, 1975. questioned the validity of the concept that As indicated in the previous two-part the tensor tympani and the stapedius mus- report, it is imperative to consider not only cles provide protection to the inner ear the palate but all of the oral-facial-pharyn- from loud sounds, except perhaps for geal system, both in normal and abnormal minimal protection (less than 10 dB) at low conditions, and both in the adult and in frequencies. They also cited research the developing child. Thus, this review in- which indicated that stapedius contraction cludes normal, abnormal, and develop- is more closely associated with voicing and mental studies on middle ear musculature, coughing than with acoustic stimuli, and the auditory tube, the velopharyngeal that the middle ear muscles might be in- mechanism, the tongue, the larynx, the volved in auditory tube opening. face and mandible, and blood supply and The literature reviewed for this report innervation relevant to cleft lip and palate. does not resolve all of these questions, but Though the relevance of embryology of it does add some focus for future research. the orofacial complex is obvious, it has Greisen and Neergaard (1975) used extra- been reviewed in a recently published re- tympanic phonometry to study middle ear port (Dickson, 1975) and will not be in- reflex activity and were able to demon- cluded as a separate topic in this review strate a tensor tympani reflex in response because of space limitations. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

Cranial Nerves 1, 5, 7-12
Cranial Nerve I Olfactory Nerve Nerve fiber modality: Special sensory afferent Cranial Nerves 1, 5, 7-12 Function: Olfaction Remarkable features: – Peripheral processes act as sensory receptors (the other special sensory nerves have separate Warren L Felton III, MD receptors) Professor and Associate Chair of Clinical – Primary afferent neurons undergo continuous Activities, Department of Neurology replacement throughout life Associate Professor of Ophthalmology – Primary afferent neurons synapse with secondary neurons in the olfactory bulb without synapsing Chair, Division of Neuro-Ophthalmology first in the thalamus (as do all other sensory VCU School of Medicine neurons) – Pathways to cortical areas are entirely ipsilateral 1 2 Crania Nerve I Cranial Nerve I Clinical Testing Pathology Anosmia, hyposmia: loss of or impaired Frequently overlooked in neurologic olfaction examination – 1% of population, 50% of population >60 years Aromatic stimulus placed under each – Note: patients with bilateral anosmia often report nostril with the other nostril occluded, eg impaired taste (ageusia, hypogeusia), though coffee, cloves, or soap taste is normal when tested Note that noxious stimuli such as Dysosmia: disordered olfaction ammonia are not used due to concomitant – Parosmia: distorted olfaction stimulation of CN V – Olfactory hallucination: presence of perceived odor in the absence of odor Quantitative clinical tests are available: • Aura preceding complex partial seizures of eg, University of Pennsylvania Smell temporal lobe origin -

New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals
RESEARCH ARTICLE Muscle Logic: New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals Robert E. Druzinsky1*, James P. Balhoff2, Alfred W. Crompton3, James Done4, Rebecca Z. German5, Melissa A. Haendel6, Anthony Herrel7, Susan W. Herring8, Hilmar Lapp9,10, Paula M. Mabee11, Hans-Michael Muller4, Christopher J. Mungall12, Paul W. Sternberg4,13, a11111 Kimberly Van Auken4, Christopher J. Vinyard5, Susan H. Williams14, Christine E. Wall15 1 Department of Oral Biology, University of Illinois at Chicago, Chicago, Illinois, United States of America, 2 RTI International, Research Triangle Park, North Carolina, United States of America, 3 Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, United States of America, 4 Division of Biology and Biological Engineering, M/C 156–29, California Institute of Technology, Pasadena, California, United States of America, 5 Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio, United States of America, 6 Oregon Health and Science University, Portland, Oregon, ’ OPEN ACCESS United States of America, 7 Département d Ecologie et de Gestion de la Biodiversité, Museum National d’Histoire Naturelle, Paris, France, 8 University of Washington, Department of Orthodontics, Seattle, Citation: Druzinsky RE, Balhoff JP, Crompton AW, Washington, United States of America, 9 National Evolutionary Synthesis Center, Durham, North Carolina, Done J, German RZ, Haendel MA, et al. (2016) United States of America, 10 Center for Genomic and Computational Biology, Duke University, Durham, Muscle Logic: New Knowledge Resource for North Carolina, United States of America, 11 Department of Biology, University of South Dakota, Vermillion, South Dakota, United States of America, 12 Genomics Division, Lawrence Berkeley National Laboratory, Anatomy Enables Comprehensive Searches of the Berkeley, California, United States of America, 13 Howard Hughes Medical Institute, M/C 156–29, California Literature on the Feeding Muscles of Mammals. -

Making Faces
Making Faces Chris Landreth CSC2529, Session 4 31 January 2011 AU1,2 (Frontalis): 2 AU4 (Corrugator): 1 AU5 (Levitor Palpabrae): 3 AU6,44 (Orbicularis Oculi): 6 How AU9 (Alaeque Nasi Labius Superioris): 1 AU10 (Labius Superioris): 3 many AU12 (Zygomatic Major): 3 letters in AU14 (Buccinator): 3 AU15 (Triangularis): 3 this AU16 (Labius Inferioris): 1 alphabet? AU17 (Mentalis): 1 AU18 (Incisivus): 1 AU20 (Risorius/Platysma): 3 AU22,23 (Orbicularis Oris): 6 AU26 (Jaw): 4 _________________________________________________ TOTAL: 41 AU’s Putting the letters together into words: Expressions The six fundamental expressions: 1. Anger 2. Sadness 3. Disgust 4. Surprise 5. Fear 6. Happiness The six fundamental expressions: 1. Anger 2. Sadness 3. Disgust 4. Surprise 5. Fear 6. Happiness A Few Words of Anger Glaring: A Few Words of Anger Glaring: Slight creases in the middle brow (Currogator) Eyelids are slightly raised (Levitor Palpabrae) Lips are clenched backward (Buccinator) Slight downturn in lip corners (Triangularis) A Few Words of Anger Miffed: A Few Words of Anger Miffed: Classic, angry ‘v-shaped’ eyebrows (Currogator) Nasolabial fold deepens, Upper lip is squared off (A.N. Labius Superioris) Lower lip raises into a pout, Dimpling in the chin (Mentalis) A Few Words of Anger Pissed off: A Few Words of Anger Pissed off: Brow raises slightly (Frontalis) Sharper Nasolabial Fold, Raised upper lip (A.N. Labius Superioris) Lower lip juts out (Orb. Oris, Lower Lip out) A Few Words of Anger Very Pissed off: A Few Words of Anger Very Pissed off: Slight squinting (Orb. Oculi) Bared upper teeth (Orb. Oris, Upper Lip Out) Squared lower lip corners, Sharp tendon creases in her neck (Risorius/Platysma) A Few Words of Anger Consumed in Rage: A Few Words of Anger Consumed in Rage: Intense, asymmetrical squinting (Orb. -

Chin Ptosis: Classification, Anatomy, and Correction/Garfein, Zide 3
Chin Ptosis: Classification, Anatomy, and Correction Evan S. Garfein, M.D.,1 and Barry M. Zide, D.M.D., M.D.1 ABSTRACT For years, the notion of chin ptosis was somehow integrated with the concept of witch’s chin. That was a mistake on many levels because chin droop has four major causes, all different and with some overlap. With this article, the surgeon can quickly diagnose which type and which therapeutic modality would work best. In some cases the problem is a simple fix, in others the droop can only be stabilized, and in the final two, definite corrective procedures are available. Of note, in certain situations two types of chin ptosis may overlap because both the patient and the surgeon may each contribute to the problems. For example, in dynamic ptosis, a droop that occurs with smile in the unoperated patient can be exacerbated and further produced by certain surgical methods also. This paper classifies the variations of the problems and explains the anatomy with the final emphasis on long-term surgical correction, well described herein. This article is the ninth on this subject and a review of them all would be helpful (greatly) for understanding the enigmas of the lower face. KEYWORDS: Lip incompetence, chin ptosis, witch’s chin, chin droop, mentalis muscle All chin ptosis patients are not alike. The abnormal anatomy, diagnosis, and management of the proper diagnosis of the type of chin ptosis places the four types of chin ptosis, as well as how to manage patient into one of four categories, which will deter- dynamic ptosis in the presence of other problems— mine who can and who cannot be helped and by which surgeon-caused or not. -

Appendix B: Muscles of the Speech Production Mechanism
Appendix B: Muscles of the Speech Production Mechanism I. MUSCLES OF RESPIRATION A. MUSCLES OF INHALATION (muscles that enlarge the thoracic cavity) 1. Diaphragm Attachments: The diaphragm originates in a number of places: the lower tip of the sternum; the first 3 or 4 lumbar vertebrae and the lower borders and inner surfaces of the cartilages of ribs 7 - 12. All fibers insert into a central tendon (aponeurosis of the diaphragm). Function: Contraction of the diaphragm draws the central tendon down and forward, which enlarges the thoracic cavity vertically. It can also elevate to some extent the lower ribs. The diaphragm separates the thoracic and the abdominal cavities. 2. External Intercostals Attachments: The external intercostals run from the lip on the lower border of each rib inferiorly and medially to the upper border of the rib immediately below. Function: These muscles may have several functions. They serve to strengthen the thoracic wall so that it doesn't bulge between the ribs. They provide a checking action to counteract relaxation pressure. Because of the direction of attachment of their fibers, the external intercostals can raise the thoracic cage for inhalation. 3. Pectoralis Major Attachments: This muscle attaches on the anterior surface of the medial half of the clavicle, the sternum and costal cartilages 1-6 or 7. All fibers come together and insert at the greater tubercle of the humerus. Function: Pectoralis major is primarily an abductor of the arm. It can, however, serve as a supplemental (or compensatory) muscle of inhalation, raising the rib cage and sternum. (In other words, breathing by raising and lowering the arms!) It is mentioned here chiefly because it is encountered in the dissection. -

Gender Differences in Genioglossus Muscle Response to the Change in Pharyngeal Airway Patency Jeffrey J
University of Connecticut OpenCommons@UConn SoDM Masters Theses School of Dental Medicine June 2001 Gender Differences in Genioglossus Muscle Response to the Change in Pharyngeal Airway Patency Jeffrey J. Blasius Follow this and additional works at: https://opencommons.uconn.edu/sodm_masters Recommended Citation Blasius, Jeffrey J., "Gender Differences in Genioglossus Muscle Response to the Change in Pharyngeal Airway Patency" (2001). SoDM Masters Theses. 21. https://opencommons.uconn.edu/sodm_masters/21 GENDER DIFFERENCES IN GENIOGLOSSUS MUSCLE RESPONSE TO THE CHANGE IN PHARYNGEAL AIRWAY PATENCY Jeffrey J. Blasius B.S., University of Connecticut, 1994 D.M.D., University of Connecticut, 1998 A Thesis Submitted in Partial Fulfillment ofthe Requirements for the Degree of Master ofDental Science at the University of Connecticut 2001 APPROVAL PAGE Master of Dental Science Thesis GENDER DIFFERENCES IN GENIOGLOSSUS MUSCLE SPONSE TO THE CHANGE IN PHARYNGEAL AIRWAY PATENCY Presented by Jeffrey J. B lasius, D.M.D. Major Advisor Dr. Eung Kwon- Pae Associate Advisor en Godwin Associate Advisor Dr. Ravindra Nanda University of Connecticut 2001 ABSTRACT Differences in genioglossal (GG) basal activity and in GG muscle response to the change in pharyngeal patency were studied using bipolar surface EMG electrodes in age- matched, historically healthy, 16 males and 15 females. To minimize hormonal influences on ventilatory activity, all female EMG records were obtained during the follicular phase of the menstrual cycle or during the placebo days for individuals using oral contraceptives. Two experimental conditions were applied: a miniature balloon was placed in the retroglossal pharynx, and three positive airway pressures were applied to the nose to observe the change in GG basal tone. -

The Muscular System Text © the Mcgraw−Hill Physiology: the Unity of Companies, 2003 Form and Function, Third Edition
Saladin: Anatomy & 10. The Muscular System Text © The McGraw−Hill Physiology: The Unity of Companies, 2003 Form and Function, Third Edition CHAPTER 10 The Muscular System Muscles of the thigh to upper calf (MRI) CHAPTER OUTLINE The Structural and Functional Organization of Muscles Acting on the Shoulder and Upper Muscles 326 Limb 352 INSIGHTS • The Functions of Muscles 326 • Muscles Acting on the Scapula 352 • Connective Tissues of a Muscle 326 • Muscles Acting on the Humerus 356 10.1 Medical History: Discovery of a • General Anatomy of Skeletal Muscles 328 • Muscles Acting on the Forearm 357 New Muscle 342 • Coordinated Action of Muscle Groups 328 • Muscles Acting on the Wrist and Hand 361 10.2 Clinical Application: Heavy Lifting • Intrinsic and Extrinsic Muscles 329 and Back Injuries 349 • Muscle Innervation 329 Muscles Acting on the Hip and Lower 10.3 Clinical Application: Hernias 351 • How Muscles Are Named 330 Limb 369 10.4 Clinical Application: Carpal • A Learning Strategy 330 • Muscles Acting on the Hip and Femur 369 Tunnel Syndrome 365 • Muscles Acting on the Knee 373 10.5 Clinical Application: Muscles of the Head and Neck 330 • Muscles Acting on the Foot 374 Intramuscular Injections 366 • Muscles of Facial Expression 330 10.6 Clinical Application: Athletic Connective Issues 387 • Muscles of Chewing and Swallowing 335 Injuries 386 • Muscles Acting on the Head 343 Chapter Review 388 Muscles of the Trunk 345 • Muscles of Respiration 345 • Muscles of the Abdomen 346 • Muscles of the Back 347 • Muscles of the Pelvic Floor 350 Brushing Up To understand this chapter, it is important that you understand or brush up on the following concepts: • Gross anatomy of the skeleton (chapter 8) • Movements of synovial joints (pp. -

Principles of Anatomy and Physiology
PRINCIPLES OF ANATOMY AND PHYSIOLOGY Tenth Edition Volume 2 Support and Movement of the Human Body Gerard J. Tortora Bergen Community College Sandra Reynolds Grabowski Purdue University John WiIey & Sons, Inc. .... , " '.. j' .. I' Brief Table of Contents ! jl : I1 11 , n il Preface IV Acknowledgements XVI To the Student XVIII Unit 1 Chapter 1 An Introduction to the Human Body 1 Organization of 2 The Chemical Level of Organization 26 the Human Body 3 The Cellular Level of Organization 59 4 The Tissue Level of Organization 103 5 The Integumentary System 139 Unit2 Chapter 6 The .Skeletal System: BoneTissue 161 Principles of Support 7 The Skeletal System:The Axial.Skeleton 185 and Movement 8 The Skeletal System:The Appendicular Skeleton 218 9 Joints 243 10 Muscle Tisuue .273 11 The Muscular System 308 Unit3 Chapter 12 Nervous Tissue 385 Control Systems of 13 The Spinal Cord and Spinal Nerves 419 the Human Body 14 The Brain and Cranial Nerves 451 15 Sensory, Motor and Integrative Systems 498 16 The Special Senses 526 17 The Autonomic Nervous System 565 18 The Endocrine System 586 Unit4 Chapter 19 The Cardiovascular System: The Blood 633 Maintenance of 20 The Cardiovascular System: The Heart 659 I the Human Body 21 The Cardiovascular System: Blood Vessels and Hemodynamics 696 22 The Lymphatic and Immune System and Resistance to Disease 764 - I 23 The Respiratory System 805 24 The Digestive System 851 25 Metabolism 906 26 The Urinary System 948 27 Fluid, Electrolyte, and Acid-Base Homeostasis 991 Unit 5 Chapter 28 The Reproductive Systems 1011