Investigation of the Effect of Organic Working Fluids on Thermodynamic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

Novel Hot Air Engine and Its Mathematical Model – Experimental Measurements and Numerical Analysis
POLLACK PERIODICA An International Journal for Engineering and Information Sciences DOI: 10.1556/606.2019.14.1.5 Vol. 14, No. 1, pp. 47–58 (2019) www.akademiai.com NOVEL HOT AIR ENGINE AND ITS MATHEMATICAL MODEL – EXPERIMENTAL MEASUREMENTS AND NUMERICAL ANALYSIS 1 Gyula KRAMER, 2 Gabor SZEPESI *, 3 Zoltán SIMÉNFALVI 1,2,3 Department of Chemical Machinery, Institute of Energy and Chemical Machinery University of Miskolc, Miskolc-Egyetemváros 3515, Hungary e-mail: [email protected], [email protected], [email protected] Received 11 December 2017; accepted 25 June 2018 Abstract: In the relevant literature there are many types of heat engines. One of those is the group of the so called hot air engines. This paper introduces their world, also introduces the new kind of machine that was developed and built at Department of Chemical Machinery, Institute of Energy and Chemical Machinery, University of Miskolc. Emphasizing the novelty of construction and the working principle are explained. Also the mathematical model of this new engine was prepared and compared to the real model of engine. Keywords: Hot, Air, Engine, Mathematical model 1. Introduction There are three types of volumetric heat engines: the internal combustion engines; steam engines; and hot air engines. The first one is well known, because it is on zenith nowadays. The steam machines are also well known, because their time has just passed, even the elder ones could see those in use. But the hot air engines are forgotten. Our aim is to consider that one. The history of hot air engines is 200 years old. -

Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste
Sustainability 2014, 6, 5714-5729; doi:10.3390/su6095714 OPEN ACCESS sustainability ISSN 2071-1050 www.mdpi.com/journal/sustainability Article Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste Franco Cotana 1,†, Antonio Messineo 2,†, Alessandro Petrozzi 1,†,*, Valentina Coccia 1, Gianluca Cavalaglio 1 and Andrea Aquino 1 1 CRB, Centro di Ricerca sulle Biomasse, Via Duranti sn, 06125 Perugia, Italy; E-Mails: [email protected] (F.C.); [email protected] (V.C.); [email protected] (G.C.); [email protected] (A.A.) 2 Università degli Studi di Enna “Kore” Cittadella Universitaria, 94100 Enna, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-075-585-3806; Fax: +39-075-515-3321. Received: 25 June 2014; in revised form: 5 August 2014 / Accepted: 12 August 2014 / Published: 28 August 2014 Abstract: The Biomass Research Centre, section of CIRIAF, has recently developed a biomass boiler (300 kW thermal powered), fed by the poultry manure collected in a nearby livestock. All the thermal requirements of the livestock will be covered by the heat produced by gas combustion in the gasifier boiler. Within the activities carried out by the research project ENERPOLL (Energy Valorization of Poultry Manure in a Thermal Power Plant), funded by the Italian Ministry of Agriculture and Forestry, this paper aims at studying an upgrade version of the existing thermal plant, investigating and analyzing the possible applications for electricity production recovering the exceeding thermal energy. A comparison of Organic Rankine Cycle turbines and Stirling engines, to produce electricity from gasified poultry waste, is proposed, evaluating technical and economic parameters, considering actual incentives on renewable produced electricity. -

Developing of a Thermodynamic Model for the Performance Analysis
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 August 2018 doi:10.20944/preprints201808.0045.v1 Peer-reviewed version available at Energies 2018, 11, 2655; doi:10.3390/en11102655 Developing of a thermodynamic model for the performance analysis of a Stirling engine prototype Miguel Torres García*, Elisa Carvajal Trujillo, José Antonio Vélez Godiño, David Sánchez Martínez University of Seville-Thermal Power Group - GMTS Escuela Técnica Superior de Ingenieros Industriales Camino de los descubrimientos s/n 41092 SEVILLA (SPAIN) e-mail: [email protected] KEYWORDS Stirling engine, combustion model Abstrac The scope of the study developed by the authors consists in comparing the results of simulations generated from different thermodynamic models of Stirling engines, characterizing both instantaneous and indicated operative parameters. The elaboration of this study aims to develop a tool to guide the decision-making process regarding the optimization of both performance and reliability of developing Stirling engines, such as the 3 kW GENOA 03 unit, on which this work focuses. Initially, the authors proceed to characterize the behaviour of the aforementioned engine using two different approaches: firstly, an ideal isothermal model is used, the simplest of those available; proceeding later to the analysis by means of an ideal adiabatic model, more complex than the first one. Since some of the results obtained with the referred ideal models are far from the expected actual ones, particularly in terms of thermal efficiency, the authors propose a set of modifications to the ideal adiabatic model. These modifications, mainly related to both heat transfer and fluid friction phenomena, are intended to overcome the limitations derived from the idealization of the engine working cycle and are expected to generate results closer to the actual behaviour of the Stirling engine, despite the increase in the complexity related to the modelling and simulation processes derived from those. -
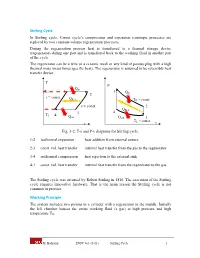
Stirling Cycle Working Principle
Stirling Cycle In Stirling cycle, Carnot cycle’s compression and expansion isentropic processes are replaced by two constant-volume regeneration processes. During the regeneration process heat is transferred to a thermal storage device (regenerator) during one part and is transferred back to the working fluid in another part of the cycle. The regenerator can be a wire or a ceramic mesh or any kind of porous plug with a high thermal mass (mass times specific heat). The regenerator is assumed to be reversible heat transfer device. T P Qin TH 1 1 Q 2 in v = const. Q Reg. TH = const. v = const. 2 QReg. 3 4 TL 4 Qout Qout 3 T = const. s L v Fig. 3-2: T-s and P-v diagrams for Stirling cycle. 1-2 isothermal expansion heat addition from external source 2-3 const. vol. heat transfer internal heat transfer from the gas to the regenerator 3-4 isothermal compression heat rejection to the external sink 4-1 const. vol. heat transfer internal heat transfer from the regenerator to the gas The Stirling cycle was invented by Robert Stirling in 1816. The execution of the Stirling cycle requires innovative hardware. That is the main reason the Stirling cycle is not common in practice. Working Principle The system includes two pistons in a cylinder with a regenerator in the middle. Initially the left chamber houses the entire working fluid (a gas) at high pressure and high temperature TH. M. Bahrami ENSC 461 (S 11) Stirling Cycle 1 TH TL QH State 1 Regenerator Fig 3-3a: 1-2, isothermal heat transfer to the gas at TH from external source. -

Stirling Engine Configuration Selection
energies Article Stirling Engine Configuration Selection Jose Egas 1 ID and Don M. Clucas 2,∗ ID 1 Department of Mechanical Engineering, University of Canterbury, Christchurch 8041, New Zealand; [email protected] 2 Department of Mechanical Engineering, University of Canterbury, Civil Mechanical E521, 20 Kirkwood Ave, Christchurch 8041, New Zealand * Correspondence: [email protected]; Tel: +64-3-364-2987 (ext. 92212) Received: 3 December 2017; Accepted: 6 February 2018; Published: 7 March 2018 Abstract: Unlike internal combustion engines, Stirling engines can be designed to work with many drive mechanisms based on the three primary configurations, alpha, beta and gamma. Hundreds of different combinations of configuration and mechanical drives have been proposed. Few succeed beyond prototypes. A reason for poor success is the use of inappropriate configuration and drive mechanisms, which leads to low power to weight ratio and reduced economic viability. The large number of options, the lack of an objective comparison method, and the absence of a selection criteria force designers to make random choices. In this article, the pressure—volume diagrams and compression ratios of machines of equal dimensions, using the main (alpha, beta and gamma) crank based configurations as well as rhombic drive and Ross yoke mechanisms, are obtained. The existence of a direct relation between the optimum compression ratio and the temperature ratio is derived from the ideal Stirling cycle, and the usability of an empirical low temperature difference compression ratio equation for high temperature difference applications is tested using experimental data. It is shown that each machine has a different compression ratio, making it more or less suitable for a specific application, depending on the temperature difference reachable. -

Stirling Engine for Solar Thermal Electric Generation
Stirling Engine for Solar Thermal Electric Generation Mike Miao He Electrical Engineering and Computer Sciences University of California at Berkeley Technical Report No. UCB/EECS-2018-15 http://www2.eecs.berkeley.edu/Pubs/TechRpts/2018/EECS-2018-15.html May 1, 2018 Copyright © 2018, by the author(s). All rights reserved. Permission to make digital or hard copies of all or part of this work for personal or classroom use is granted without fee provided that copies are not made or distributed for profit or commercial advantage and that copies bear this notice and the full citation on the first page. To copy otherwise, to republish, to post on servers or to redistribute to lists, requires prior specific permission. Acknowledgement I would like to thank Professor Seth Sanders for his mentorship and helping me to be a better engineer, for his perpetual good humor and kindness, and for always being available for his students. I would also like to thank Artin Der Minassians for his guidance and help. Thanks to Aron, Matt, Roger, Sarah, and Shai on the Cleantech-to-Market team for their research on the market viability of the technology. Thanks to Alan, Shong, Kelvin, Alex, and Samir and others for their help. Stirling Engine for Solar Thermal Electric Generation by Mike Miao He A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering { Electrical Engineering and Computer Sciences and the Designated Emphasis in Energy Science and Technology in the Graduate Division of the University -

Operation of Thermoacoustic Stirling Heat Engine Driven Large Multiple Pulse Tube Refrigerators
LA-UR-04-2820, submitted to the proceedings of the 13th Int'l Cryocooler Conf. 1 Operation of Thermoacoustic Stirling Heat Engine Driven Large Multiple Pulse Tube Refrigerators Bayram Arman, John Wollan, and Vince Kotsubo Praxair, Inc. Tonawanda, NY 14150 Scott Backhaus and Greg Swift Los Alamos National Laboratory Los Alamos, NM 87545 ABSTRACT With support from Los Alamos National Laboratory, Praxair has been developing ther- moacoustic Stirling heat engines and refrigerators for liquefaction of natural gas. The combina- tion of thermoacoustic engines with pulse tube refrigerators is the only technology capable of producing significant cryogenic refrigeration with no moving parts. A prototype, powered by a natural-gas burner and with a projected natural-gas-liquefaction capacity of 500 gal/day, has been built and tested. The unit has liquefied 350 gal/day, with a projected production efficiency of 70% liquefaction and 30% combustion of an incoming gas stream. A larger system, intended to have a liquefaction capacity of 20,000 gal/day and an efficiency of 80 to 85% liquefaction, has undergone preliminary design. In the 500 gal/day system, the combustion-powered thermoacoustic Stirling heat engine drives three pulse tube refrigerators to generate refrigeration at methane liquefaction tempera- tures. Each refrigerator was designed to produce over 2 kW of refrigeration. The orifice valves of the three refrigerators were adjusted to eliminate Rayleigh streaming in the pulse tubes. This pa- per describes the hardware, operating experience, and some recent test results. INTRODUCTION Praxair has been developing thermoacoustic liquefiers and refrigerators for liquefaction of natural gas and for other cryogenic applications. -

Lecture 3 Thermodynamic Principles of Energy Conversion
Thermodynamic principles of energy conversion Md. Mizanur Rahman School of Mechanical Engineering Universiti Teknologi Malaysia 1 Introduction • Mechanical and electrical power developed from the combustion of fossil fuels or the fission of nuclear fuel or renewable sources • This released energy is never lost but is transformed into other forms • This conservation of energy is explicitly expressed by the first law of thermodynamics • The work of an engine cannot be the same as the energy available in the fuel sources • Second law of thermodynamics is to meet to happen the process 2 Forms of energy • Energy can exist in numerous forms such as thermal, mechanical, kinetic, potential, electric, magnetic, chemical, and nuclear, and their sum constitutes the total energy, E of a system. • Mechanical Energy • Kinetic energy, KE: The energy that a system possesses as a result of its motion relative to some reference frame, KE=1/2mV2. • Potential energy, PE: The energy that a system possesses as a result of its elevation in a gravitational field, PE=mgh • For a moving body, E=PE+KE remains constant 3 Internal energy, U: The sum of all the microscopic forms of energy. • For gases, molecules are so widely separated in space that they may be considered to be moving independently of each other, each possessing a distinct total energy. • In the case of liquids or solids, each molecule is under the influence of forces exerted by nearby molecules • Changes in internal energy are measurable by changes in temperature, pressure, and density. 4 • Chemical energy: The internal energy associated with the atomic bonds in a molecule. -

The Stirling Cycle the Ultimate Way to Generate Cooling Power
The Stirling Cycle The ultimate way to generate cooling power History The Stirling cycle is a thermodynamic closed cycle invented in 1816 by the Scottish minister Robert Stirling. It was used as an engine and was considered at the time to be capable of replacing the steam engine since their steam boilers were prone to life- threatening explosions. The counterpart of the Stirling engine, the refrigerator, was first recognized in 1832. Both machines experienced high and low points during the nineteenth century. The principle behind the machines was almost condemned to obscurity after the invention of the internal combustion engine (gas-, petrol-, and diesel motors) and compressor refrigerators with external evaporation. In 1938 the famous Dutch Philips Research Laboratory was looking for a means to power electricity generators for short wave communication systems in remote areas without electricity supply. The practically-forgotten Stirling engine attracted their attention. In 1946 Philips started optimizing the Stirling cycle to be used for cryogenic cooling. The result was the development of the world conquering Stirling Cryogenerator, marking the start of significant cryogenic activities at Philips. Though the Stirling engine itself never became a commercial success, the Stirling Cryogenerator has been selling by thousands worldwide and has been incorporated in equipment and projects used from Antarctica to the North Pole. Today In the beginning of the 90’s the Philips Cryogenic Division became independent and continued its worldwide activities under the name of Stirling Cryogenics BV. Since 2014, DH Industries BV incorporated the Stirling Cryogenics products as a brand alongside its CryoZone brand. DH Industries is the only company in the world that successfully produces Stirling cycle- based cryogenerators with cooling powers of 1,000-4,000 watt (at 77K) per unit. -

Schmidt Analysis for Stirling Engines
SCHMIDT ANALYSIS FOR STIRLING ENGINES This Analysis was written by David Berchowitz and Israel Urieli and published in their book "Stirling Cycle Engine Analysis". The analysis was written for engines but applies also to coolers. The difference between the two analysis is that the Rejector (k) in the engine is the lower temperature reject sink and in the cooler is the higher temperature reject sink. The Acceptor is the heat accept in both engine and cooler but corresponds to the lower temperature in the cooler and to the higher temperature in the engine. Nomenclature Subscripts T Temperature c Compression Space P Pressure e Expansion Space M Mass r Regenerator R Gas Constant k Rejector V Volume h Acceptor W Work sw swept A.1 The Schmidt analysis A.1.1 Background The apparent conceptual simplicity of the Stirling engine belies its intractability to mathematical analysis. The difficulty of describing even idealized models of the engine in terms of simple closed-form equations is one of the primary reasons for the widespread skepticism and lack of understanding which exists even today. In Chapter 2 we derived the basic set of equations which describe the Ideal Isothermal model (table 2.1). Gustav Schmidt of the German Polytechnic Institute of Prague published an analysis in 1871 in which he obtained closed- form solutions of these equations for the special case of sinusoidal volume variations of the working spaces (Schmidt 1871). This analysis is still used today as the classic Stirling cycle analysis. It was derived in order to describe the highly successful Lehmann engine shown in figure A. -

Progress of Stirling Cycle Analysis and Loss Mechanism Characterization
DOE/NASA/SO112-67 iW5A iivi-88897 Progress of Stirling Cycle Analysis and Loss Mechanism Characterization [NASA-TPl-8889 1) PROGFESS OF STIRLING CYCLE N87-13359 ANALYSIS AED LOSS NECFANISM CHARACTEEIZATION Einal Report (NASA) 19 p CSCL 10B Unclas ~1185 44720 Roy C. Tew, Jr. National Aeronautics and Space Administration Lewis Rzsearch Center Work performed for U.S. DEPARTMENT OF ENERGY Conservation and Renewable Energy Office of Vehicle and Engine R&D It \ Prepared for f Twenty-fourth Automotive Technology Development a sponsored by Society of Automotive Engineers Dearborn, Michigan, October 27-30, 1986 . DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Printed in the United States of America Available from National Technical Information Service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA 22161 NTlS price codes1 Printed copy: A02 Microfiche copy: A01 %odes are used for pricing all publications.