Case of Neuronal Intranuclear Inclusion Disease with Dynamic Perfusion Changes Lacking Typical Signs on Diffusion-Weighted Imagi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fact Book 2020(英語版)All.Indd
with Other Universities Overview and Comparisons Overview 13. Income and Expenditure 13-1. Budgeted Income and Expenditure Number of Faculty Number of Faculty and Staff Members Kyushu Universityʼs budgeted income and expenditures have been on a downward trend since FY 2018, the year the campus relocation was completed. ◆Kyushu University◆ Number of Students Budgeted Income and Expenditure (¥million) 148,822 150,000 139,617 Admissions 133,429 133,160 148,822 128,240 129,470 139,617 125,852 126,898 133,429 133,160 115,593 129,470 128,240 125,852 126,898 115,593 100,000 Enrollment Conferred 50,000 Graduate Degrees Degrees Graduate 0 Career Paths and Career Paths Employment Status Employment 2012 2013 2014 2015 2016 2017 2018 2019 2020 (FY) Budgeted income Budgeted expenditure Examinations Success in Qualification *Source: Kyushu University Information ◆Other Universities (FY2019) ◆ Research Budgeted Income and Expenditure The University 261,172 of Tokyo 261,172 International Kyoto 177,863 University 177,863 Osaka 157,952 University 157,952 Industry-University- Tohoku 146,961 Government Collaboration Government University 146,961 Kyushu 125,852 University 125,852 Nagoya 106,118 University Hospital University University 106,118 Hokkaido 100,552 University 100,552 Income and Expenditure 0 50,000 100,000 150,000 200,000 250,000 300,000 Income and Expenditure (¥million) Budgeted income Budgeted expenditure *Source: Websites and Information for each university Education Programs Selection of Research/ 174 KYUSHU UNIVERSITY FACT BOOK 2020 Overview -
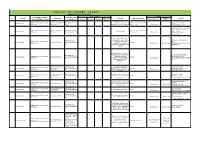
Robotics Laboratory List
Robotics List (ロボット技術関連コースのある大学) Robotics List by University Degree sought English Undergraduate / Graduate Admissions Office No. University Department Professional Keywords Application Deadline Degree in Lab links Schools / Institutes or others Website Bachelor Master’s Doctoral English Admissions Master's English Graduate School of Science and Department of Mechanical http://www.se.chiba- Robotics, Dexterous Doctoral:June and December ○ http://www.em.eng.chiba- 1 Chiba University ○ ○ ○ Engineering Engineering u.jp/en/ Manipulation, Visual Recognition Master's:June (Doctoral only) u.jp/~namiki/index-e.html Laboratory Innovative Therapeutic Engineering directed by Prof. Graduate School of Science and Department of Medical http://www.tms.chiba- Doctoral:June and December ○ 1 Chiba University ○ ○ Surgical Robotics ○ Ryoichi Nakamura Engineering Engineering u.jp/english/index.html Master's:June (Doctoral only) http://www.cfme.chiba- u.jp/~nakamura/ Micro Electro Mechanical Systems, Micro Sensors, Micro Micro System Laboratory (Dohi http://global.chuo- Graduate School of Science and Coil, Magnetic Resonance ○ ○ Lab.) 2 Chuo University Precision Mechanics u.ac.jp/english/admissio ○ ○ October Engineering Imaging, Blood Pressure (Doctoral only) (Doctoral only) http://www.msl.mech.chuo-u.ac.jp/ ns/ Measurement, Arterial Tonometry (Japanese only) Method Assistive Robotics, Human-Robot Communication, Human-Robot Human-Systems Laboratory http://global.chuo- Graduate School of Science and Collaboration, Ambient ○ http://www.mech.chuo- 2 Chuo University -

Semantic Integration of User Data-Models and Processes
Program Committee CANDAR 2016 Track 1: Algorithms and Applications Chair: Akihiro Fujiwara, Kyushu Institute of Technology, Japan Stéphane Devismes, VERIMAG UMR 5104, France Man Duhu, Shenzhen Institutes of Advanced Technology, China Masaru Fukushi, Yamaguchi University, Japan Teofilo Gonzalez, University of California, USA Qianping Gu, Simon Fraser University, Canada Xin Han, Dalian University of Technology, China Fumihiko Ino, Osaka University, Japan Hirotsugu Kakugawa, Osaka University, Japan Sayaka Kamei, Hiroshima University, Japan Christos Kartsaklis, Oak Ridge National Laboratory, USA Yosuke Kikuchi, Tsuyama National College of Technology, Japan Yonghwan Kim, Nagoya Institute of Technology, Japan Guoqiang Li, Shanghai Jiao Tong University, China Yamin Li, Hosei University, Japan Ami Marowka, Bar-Ilan University, Israel Eiji Miyano, Kyushu Institute of Technology, Japan Hiroaki Mukaidani, Hiroshima University, Japan Takayuki Nagoya, Tottori University of Environmental Studies, Japan Fukuhito Ooshita, NAIST, Japan Ke Qiu, Brock University, Canada Wei Sun, Data61/CSIRO, Australia Daisuke Takafuji, Hiroshima University, Japan Satoshi Taoka, Hiroshima University, Japan Jerry Trahan, Louisiana State University, USA Yushi Uno, Osaka Prefecture University, Japan Shingo Yamaguchi, Yamaguchi University, Japan Hiroshi Yamamoto, Ritsumeikan University, Japan Track 2: Architecture and Computer System Chair: Michihiro Koibuchi, National Institute of Informatics, Japan Martti Forsell, VTT, Finland Ikki Fujiwara, National Institute of Informatics, -

Hicells 2020)
Hawaiʻi International Conference on English Language and Literature Studies (HICELLS 2020) “Trends in Research and Pedagogical Innovations in English Language and Literature” CONFERENCE PROGRAM March 13 -14, 2020 HICELLS 2020 03/13-14/2020 Hawaii International Conference on English Language and Literature Studies (HICELLS 2020) English Department University of Hawaii at Hilo March 13-14, 2020 CONFERENCE PROGRAM DAY 1 (FRIDAY) March 13, 2020 Time Venue: UCB 100 8:00 – 8:30 Conference Registration 8:30 – 8:45 Kipaepae 8:45 – 9:00 Welcome Address Dr Bonnie D. Irwin Chancellor University of Hawaii at Hilo 9:00 – 9:45 Keynote Address 1 Minds, Machines and Language: What Does the Future Hold? William O’Grady University of Hawaii at Manoa Hawaii, USA BREAK: UCB 127 9:45- 10:00 PARALLEL SESSION 1 10:00 – 12:20 Room: UCB 127 (10:00-12:20) No. Presenters Papers 1 Michio Hosaka The Emergence of Functional Projections in the Nihon University History of English 2 Susana T. Udoka A Study of English and Annang Clause Syntax: Akwa Ibom State University From the View Point of Grammaticality and Global Obio Akpa Campus Intelligibility HICELLS 2020 2 HICELLS 2020 03/13-14/2020 3 Quentin C. Sedlacek Contestation, Reification, and African American California State University English in College Linguistics Courses Monterey Bay 4 Hiromi Otaka On the Aspect Used in the Subordinate Clause of Kwansei Gakuin University “This is the first time ~” in English 5 Chiu-ching Tseng English Word Boundary Perception by Mandarin George Mason University Native Speakers 6 Yumiko Mizusawa An Analysis of Lexicogrammatical and Semantic Seijo University Features in Academic Writing by Japanese EFL Learners 7 Noriko Yoshimura Japanese EFL Learners’ Structural University of Shizuoka Misunderstanding: ECM Passives in L2 English Mineharu Nakayama The Ohio State University Atsushi Fujimori University of Shizuoka Room: UCB 101 (10:00-12:20) No. -
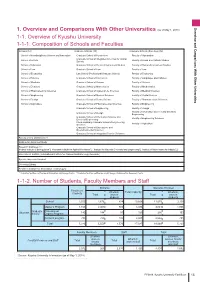
(As of Ay 1, 2018) 1-1. Overview of Kyushu University 1-1-1
Overview and Comparisons With Other Universities 1 vervie and Comarisons ith ther Universities (as of ay 1, 2018) 1-1. Overview of Kyushu University 1-1-1. Composition of Schools and Faculties Schools (12) raduate Schools (18) raduate Schools (Faculties) (16) School of Interdisciplinary Science and Innovation raduate School of Humanities Faculty of Humanities raduate School of Integrated Sciences for lobal School of etters Faculty of Social and Cultural Studies Society School of ducation raduate School of Human-nvironment Studies Faculty of Human-nvironment Studies School of aw raduate School of aw Faculty of aw School of conomics aw School (Professional raduate School) Faculty of conomics School of Science raduate School of conomics Faculty of anguages and Cultures School of edicine raduate School of Science Faculty of Science School of Dentistry raduate School of athematics Faculty of athematics School of Pharmaceutical Sciences raduate School of Systems ife Sciences Faculty of edical Sciences School of ngineering raduate School of edical Sciences Faculty of Dental Science School of Design raduate School of Dental Science Faculty of Pharmaceutical Sciences School of Agriculture raduate School of Pharmaceutical Sciences Faculty of ngineering raduate School of ngineering Faculty of Design Faculty of Information Science and lectrical raduate School of Design ngineering raduate School of Information Science and Faculty of ngineering Sciences lectrical ngineering Interdisciplinary raduate School of ngineering Faculty of Agriculture Sciences -

Lecture Notes in Physics
AUTHOR INDEX Akama, K ............ 267 Kakuto, A ............. 217 Alfraro, J .......... 65 Kasuya, N ............ 41 Arnowitt, R ......... 231 Kawai, H ............. 133 Komatsu, H ........... 217 Baekler, P .......... I Banks, T ............ 227 Matsuda, H ........... 84 Matsuda, T ........... 146 Candelas, P ......... 107 Chamseddine, A.H .... 231 Nakanishi, N ......... 171 Nambu, Y ............. 277 DeWitt, B.S ......... 189 Nath, P .............. 231 DeWitt-Morette, C. .. 166 Nishijima, K ......... 155 Nishino, H ........... 249 Eguchi, T ........... 133 Endo, J ............. 146 Ohta, N. 226 ........ 222 Ojima, I ............. 161 Fradkin, E.S ........ 284,293 Omote, M ............. 204 Freund, P.G.O ....... 259 Osakabe, N ........... 146 Fujii, Y ............ 272 Fujiwara, H ......... 146 Parker, L ........... 89,96 Fukuda, R ........... 58 Fukuda, T ........... 84 Rosen, N ............ 301 Fukuhara, A ......... 146 Fulling, S.A ........ 101 Sakai, N ....... ..... 208 Sakamoto, J ......... 113 Halpern, L .......... 26 Sakita, B ............ 65 Hehl, F.W ........... I Sasaki, R ........... 31 Horibe, M ........... 113 Sato, M ............. 75 Horwitz, G .......... 254 Seki, Y ............. 84 Hosoya, A ........... 113 Shigemitsu, J ....... 120 Shinagawa, K ........ 146 Ichinose, S ......... 204 Shirafuji, T ........ 16 Inoue, K ............ 217 Strominger, A ....... 184 Iwasaki, Y .......... 141 Sugawara, H ......... 262 Sugita, Y ........... 146 Jackiw, R ........... 45 Suura, H ............ 62 Suzuki, R ........... 146 307 Takeshita, S ........ 217 Tauber, G.E ......... 301 Tomimatsu, A ........ 36 Tonomura, A ......... 146 Townsend, P.K ....... 240 Tseytlin, A.A ....... 284,293 Umezaki, H .......... 146 Viallet, C.M ........ 116 Yokoyama, K ......... 84 Yoneya, T ........... 79 Yoshi4, T ........... 141 List of Participants Akama, K. DeWitt, B.S. Saitama Medical School Department of Physics Japan University of Texas at Austin USA Aoki, K. RIFP, Kyoto University DeWitt-Morette, C. Japan Department of Astronomy University of Texas at Austin USA Arafune, J. -

The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension
Hypertension Research (2014) 37, 254–255 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension CHAIRPERSON Kazuaki SHIMAMOTO (Sapporo Medical University) WRITING COMMITTEE Katsuyuki ANDO (University of Tokyo) Ikuo SAITO (Keio University) Toshihiko ISHIMITSU (Dokkyo Medical University) Shigeyuki SAITOH (Sapporo Medical University) Sadayoshi ITO (Tohoku University) Kazuyuki SHIMADA (Jichi Medical University) Masaaki ITO (Mie University) Kazuaki SHIMAMOTO (Sapporo Medical University) Hiroshi ITOH (Keio University) Tatsuo SHIMOSAWA (University of Tokyo) Yutaka IMAI (Tohoku University) Hiromichi SUZUKI (Saitama Medical University) Tsutomu IMAIZUMI (Kurume University) Norio TANAHASHI (Saitama Medical University) Hiroshi IWAO (Osaka City University) Kouichi TAMURA (Yokohama City University) Shinichiro UEDA (University of the Ryukyus) Takuya TSUCHIHASHI (Steel Memorial Yahata Hospital) Makoto UCHIYAMA (Uonuma Kikan Hospital) Mitsuhide NARUSE (NHO Kyoto Medical Center) Satoshi UMEMURA (Yokohama City University) Koichi NODE (Saga University) Yusuke OHYA (University of the Ryukyus) Jitsuo HIGAKI (Ehime University) Katsuhiko KOHARA (Ehime University) Naoyuki HASEBE (Asahikawa Medical College) Hisashi KAI (Kurume University) Toshiro FUJITA (University of Tokyo) Naoki KASHIHARA (Kawasaki Medical School) Masatsugu HORIUCHI (Ehime University) Kazuomi KARIO (Jichi Medical University) Hideo MATSUURA (Saiseikai Kure Hospital) -

12. International Law and Organizations
DEVELOPMENTS IN 2006 ― ACADEMIC SOCIETIES 119 (1)“The idea and reality of ‘European Social Model” Misako Nishio(Ph.D. Candidate, Graduate Schools for Law and Politics University of Tokyo) (2)“The Possibility for Direct Democracy in the European Communities” Yuko Hosoi(Lecturer, Hosei University) (3)“EU’s Southeastward Enlargement and Challenges for the Western Balkans” Yoji Koyama(Niigata University) 12. International Law and Organizations I. The Japanese Society of International Law held its 2006 Spring Session at Okayama University on May 13, 2006. (1)“The Doctrine of Necessity in International Law: Its Historical Development and Contemporary Problems” Takuhei Yamada(Associate Professor, Kobe Gakuin University) (2)“Rules of International Law on Civil Jurisdiction” Tadashi Kanzaki(Professor, Gakushuin University) (3)“Factors Affecting Asian FTA Practices: An Approach to Surmount Difficulties” Chang- fa Lo(Professor, National Taiwan University) (4)“Changing the Legal Text of the WTO Agreement: International Negotiations and National Implementations” Tomohiko Kobayashi(Deputy Director, Ministry of Economy, Trade and Industry) (5)“The Development and Significance of Regional Economic Integration” Akio Shimizu(Professor, Waseda University) II. The Japanese Society of International Law held its 2006 Fall Session at Yokohama National University on October 7 and 8, 2006. Day One: 120 WASEDA BULLETIN OF COMPARATIVE LAW Vol. 26 (1)“The ‘Follow- Up’ by the International Law Commission” Tetsuya Nakano(Associate Professor, Kansai University) (2)“The -

Allantoin, a Stress-Related Purine Metabolite, Can Activate Jasmonate Signaling in a MYC2-Regulated and Abscisic Acid-Dependent Manner
Journal of Experimental Botany, Vol. 68, No. 17 p. 5011, 2017 doi:10.1093/jxb/erw289 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details) CORRIGENDUM Allantoin, a stress-related purine metabolite, can activate jasmonate signaling in a MYC2-regulated and abscisic acid-dependent manner Hiroshi Takagi1, Yasuhiro Ishiga2, Shunsuke Watanabe1,*, Tomokazu Konishi3, Mayumi Egusa4, Nobuhiro Akiyoshi1, Takakazu Matsuura5, Izumi C. Mori5, Takashi Hirayama5, Hironori Kaminaka4, Hiroshi Shimada1 and Atsushi Sakamoto1,† 1 Graduate School of Science, Hiroshima University, Higashi-Hiroshima 739-8526, Japan 2 Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba 305-8572, Japan 3 Faculty of Bioresource Sciences, Akita Prefectural University, Akita 010-0195, Japan 4 Faculty of Agriculture, Tottori University, Tottori 680-8553, Japan 5 Institute of Plant Science and Resources, Okayama University, Kurashiki 710-0046, Japan * Present address: RIKEN Center for Sustainable Resource Science, Yokohama 230-0045, Japan † Correspondence: E-mail, [email protected] Journal of Experimental Botany, Vol. 67, No. 8, pp. 2519–2532, 2016, doi: 10.1093/jxb/erw071. In Figure 6B of the above paper, the real-time reverse-transcription quantitative PCR data for JAZ3 were inadvertently used to represent the relative expression of both JAZ3 and MYC2. The correct data for MYC2 are provided in the revised version of this figure shown below. This correction does not affect the interpretation of the results or the conclusions of this work. © The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.. -

Transferring to TD2 in April 2021)
Classification ⑪ For current students admitted in September (M2, second year of the integrated doctoral program) PEP WISE Program Application Requirements Application for the 4th WISE Program (Transferring to TD2 in April 2021) Program Overview The Graduate Program for Power Energy Professionals (PEP), Waseda University from MEXT WISE Program (Doctoral Program for World-leading Innovative & Smart Education) is an integrated 5-year doctoral professional program delivered in collaboration with 13 universities (Waseda University, Hokkaido University, Tohoku University, University of Fukui, University of Yamanashi, Tokyo Metropolitan University, Yokohama National University, Nagoya University, Osaka University, Hiroshima University, Tokushima University, Kyushu University, and University of the Ryukyus). In addition to their specialization and degree, students are awarded a PEP Excellence Doctoral Program Completion Certificate upon satisfying the requirements for the program (45 credits over 5 years). WISE compulsory courses (seven courses for ten credits) are offered at Waseda University, while WISE comprehensive elective courses are offered at the students’ own program department. WISE compulsory courses are designed in consideration of students from all participating universities, and are offered in multiple formats, including on-demand; several-day, stay-overnight, intensive-study sessions; and practicums at collaborating organizations. To complete the PEP training program, students must earn a doctoral degree in their specialization. Hence, students must meet their own university program’s requirements. 1. Applicant Qualifications ◆ Common for all participating universities - At the time of application, the applicant must be an officially registered second-year master’s program student at a participating university. - The applicant must have accumulated 20 credits or more (including the courses currently in progress) of the WISE comprehensive elective courses and the WISE general elective courses specific to the specialization during the master’s program. -

N E W S L E T T
AATJNEWSLETTER VOL. 7, NO. 3 PRESIDENT’S MESSAGE み な さ ん 、夏 は い か が で ーシップ研修そして、J―CANワークショップを10月 したか。働き続けていた 5−7日の週末にインディアナにて行います。今回の焦 方 も 、ほ と ん ど 休 ん で い 点となるのは、中西部地域の日本語教育です。近くに たという方も、何れにし 住んでいる方は、ぜひ参 加してください。アドボカシ ても、充実した時間を過 ーについては、これで終わりということはなく、常にア ごせたと希 望していま ドボカシーの気持ちを忘れずにいなくてはいけませ す。 ん。その活動は、例えば国会議員に手紙を書くといっ た政治色の濃いものもあれば、次世代の教師になる Suwako Watanabe 前 回 のメッセ ージ にも書 ように生徒を励ますなど、様々な形が考えられます。 渡辺素和子 き ま し た が 、8 月 の 初 め にイタリア の ベ ネチアで ACTFL学会実行委員の知念先生、田中先生、魚立 開催された ICJLE – International Conference 先生は、準備のための最終段階に入りつつありま on Japanese Language Education に出席して す。11月のニューオーリンズでのACTFL学会で、多 きました。基調講演をされた立教大学の鳥飼玖美子 くの会員方々の参加を期待しています。 先生は、言語では社会言語や語用論といった面に内 在する「見えない文化」の重要性を強調していまし 会長 渡辺素和子 た。また、イギリスからの基調講演者であるエイドリ アン・ホリデー先生は、L2とC2が全く同等のものと する考え方に疑問を唱え、間文化性の複雑なプロセ How was your summer? I hope it was productive スについて語っておられました。発表者や司会者がし and meaningful whether you were working or ばしば触れていた本学会のテーマ、「平和への対話」 vacationing. には色々考えさせられることが多く、学会中、常に、 教室現場の日本語指導がどのように「平和」につなが As I mentioned in my previous message, I went るだろうかと、自問自答していました。そういう意味 to Venice, Italy to attend the ICJLE (International で、大きな視野で日本語教育を見つめ直すいい機会 Conference on Japanese Language Education) in となりました。 early August. Professor Emeritus Kumiko Torikai, Rikkyo University, gave a keynote speech in which ICJLEでは、たくさんの国からの日本語教育者が代 she emphasized the importance of teaching the 表として集まり、プロジェクトや問題を報告し合いま ‘invisible culture’ that is reflected in sociolinguistic した。日本語学習者数では、イタリアは、ドイツ、フラ and pragmatic aspects of language. The second ンスに次いで、第3位だそうです。またイギリスは、EU keynote speaker, Professor Adrian Holliday, 離脱の影響で、ヨーロッパからの留学生が減少する Canterbury Christ Church University, UK, と い う 懸 念 が あ る と 聞 き ま し た 。や は り 、教 育 は 、政 challenged the notion of “L2 equates C2,” and 治の影響を受けざるを得ないようです。 reminded us how complicated the development of interculturality is. -

CICE Policy Brief
CICE Policy Brief Forewords Kazuo Kuroda, Waseda University, Japan 1. Reconsidering the meaning of inclusive education Yukari Ishida, JICA Hokkaido, Japan 1 2. An exploration of education experiences and identity construction of blind students in the United Kingdom Yukari Ishida, JICA Hokkaido, Japan 11 3. Vernacular Inclusive Education: ‘Reasonable Accommodation’ in Viet Nam Kengo, Shirogane, Biwako Gakuin University, Japan 21 4. Inclusive education in Singapore: From the perspectives of efficiency versus equity Yuko Nonoyama-Tarumi, Musashi University, Japan 35 5. Resource room inclusive education in India at a crossroads: A case study of Chennai, South India Tatsuya Kusakabe, Hiroshima University, Japan, Robinson Tamburaj Madras Christian College, India 47 6. Inclusive education in Bhutan Riho Sakurai, Hiroshima University, Japan 53 7. Inclusive Education in Sri Lanka: Factors contributing to good practices Hiroko Furuta, Kumamoto University, Japan K. A. C. Alwis, Open University of Sri Lanka, Sri Lanka 63 8. Education for children with disabilities in Maldives: Special needs education and inclusive education Minoru Morishita, Tokyo University of Marine Science and Technology, Japan Jun Kawaguchi, University of Tsukuba, Japan 73 9. Inclusive Education in Bangladesh: Current Status, Scope and Implications for Learning Improvement Asim Das, Institute of Education and Research, University of Dhaka, Bangladesh Tatsuya Kusakabe, Center for the Study of International Cooperation in Education, Hiroshima University, Japan 81 10. The impact