Important Safety Note
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Communal Commercial Check City of Aachen
Eigentum von Fahrländer Partner AG, Zürich Communal commercial check City of Aachen Location Commune Aachen (Code: 5334002) Location Aachen (PLZ: 52062) (FPRE: DE-05-000334) Commune type City District Städteregion Aachen District type District Federal state North Rhine-Westphalia Topics 1 Labour market 9 Accessibility and infrastructure 2 Key figures: Economy 10 Perspectives 2030 3 Branch structure and structural change 4 Key branches 5 Branch division comparison 6 Population 7 Taxes, income and purchasing power 8 Market rents and price levels Fahrländer Partner AG Communal commercial check: City of Aachen 3rd quarter 2021 Raumentwicklung Eigentum von Fahrländer Partner AG, Zürich Summary Macro location text commerce City of Aachen Aachen (PLZ: 52062) lies in the City of Aachen in the District Städteregion Aachen in the federal state of North Rhine-Westphalia. Aachen has a population of 248'960 inhabitants (31.12.2019), living in 142'724 households. Thus, the average number of persons per household is 1.74. The yearly average net migration between 2014 and 2019 for Städteregion Aachen is 1'364 persons. In comparison to national numbers, average migration tendencies can be observed in Aachen within this time span. According to Fahrländer Partner (FPRE), in 2018 approximately 34.3% of the resident households on municipality level belong to the upper social class (Germany: 31.5%), 33.6% of the households belong to the middle class (Germany: 35.3%) and 32.0% to the lower social class (Germany: 33.2%). The yearly purchasing power per inhabitant in 2020 and on the communal level amounts to 22'591 EUR, at the federal state level North Rhine-Westphalia to 23'445 EUR and on national level to 23'766 EUR. -

Die Euregiobahn
Stolberg-Mühlener Bahnhof – Stolberg-Altstadt 2021 > Fahrplan Stolberg Hbf – Eschweiler-St. Jöris – Alsdorf – Herzogenrath – Aachen – Stolberg Hbf Eschweiler-Talbahnhof – Langerwehe – Düren Bahnhof/Haltepunkt Montag – Freitag Mo – Do Fr/Sa Stolberg Hbf ab 5:11 6:12 7:12 8:12 18:12 19:12 20:12 21:12 22:12 23:12 23:12 usw. x Eschweiler-St. Jöris ab 5:18 6:19 7:19 8:19 18:19 19:19 20:19 21:19 22:19 23:19 23:19 alle Alsdorf-Poststraße ab 5:20 6:21 7:21 8:21 18:21 19:21 20:21 21:21 22:21 23:21 23:21 60 Alsdorf-Mariadorf ab 5:22 6:23 7:23 8:23 18:23 19:23 20:23 21:23 22:23 23:23 23:23 Minu- x Alsdorf-Kellersberg ab 5:24 6:25 7:25 8:25 18:25 19:25 20:25 21:25 22:25 23:25 23:25 ten Alsdorf-Annapark an 5:26 6:27 7:27 8:27 18:27 19:27 20:27 21:27 22:27 23:27 23:27 Alsdorf-Annapark ab 5:31 6:02 6:32 7:02 7:32 8:02 8:32 9:02 18:32 19:02 19:32 20:02 20:32 21:02 21:32 22:02 22:32 23:32 23:32 Alsdorf-Busch ab 5:33 6:04 6:34 7:04 7:34 8:04 8:34 9:04 18:34 19:04 19:34 20:04 20:34 21:04 21:34 22:04 22:34 23:34 23:34 Herzogenrath-A.-Schm.-Platz ab 5:35 6:06 6:36 7:06 7:36 8:06 8:36 9:06 18:36 19:06 19:36 20:06 20:36 21:06 21:36 22:06 22:36 23:36 23:36 Herzogenrath-Alt-Merkstein ab 5:38 6:09 6:39 7:09 7:39 8:09 8:39 9:09 18:39 19:09 19:39 20:09 20:39 21:09 21:39 22:09 22:39 23:39 23:39 Herzogenrath ab 5:44 6:14 6:44 7:14 7:44 8:14 8:44 9:14 18:44 19:14 19:44 20:14 20:45 21:14 21:44 22:14 22:44 23:43 23:43 Kohlscheid ab 5:49 6:19 6:49 7:19 7:49 8:19 8:49 9:19 18:49 19:19 19:49 20:19 20:50 21:19 21:49 22:19 22:49 23:49 23:49 Aachen West ab 5:55 6:25 6:55 -

Family Businesses in Germany and the United States Since
Family Businesses in Germany and the United States since Industrialisation A Long-Term Historical Study Family Businesses in Germany and the United States since Industrialisation – A Long-Term Historical Study Industrialisation since States – A Long-Term the United and Businesses Germany in Family Publication details Published by: Stiftung Familienunternehmen Prinzregentenstraße 50 80538 Munich Germany Tel.: +49 (0) 89 / 12 76 400 02 Fax: +49 (0) 89 / 12 76 400 09 E-mail: [email protected] www.familienunternehmen.de Prepared by: Institut für Wirtschafts- und Sozialgeschichte Platz der Göttinger Sieben 5 37073 Göttingen Germany Univ.-Prof. Dr. Hartmut Berghoff Privatdozent Dr. Ingo Köhler © Stiftung Familienunternehmen, Munich 2019 Cover image: bibi57 | istock, Sasin Tipchai | shutterstock Reproduction is permitted provided the source is quoted ISBN: 978-3-942467-73-5 Quotation (full acknowledgement): Stiftung Familienunternehmen (eds.): Family Businesses in Germany and the United States since Indus- trialisation – A Long-Term Historical Study, by Prof. Dr. Hartmut Berghoff and PD Dr. Ingo Köhler, Munich 2019, www.familienunternehmen.de II Contents Summary of main results ........................................................................................................V A. Introduction. Current observations and historical questions ..............................................1 B. Long-term trends. Structural and institutional change ...................................................13 C. Inheritance law and the preservation -

Beratungs- Und Hilfsangebote Bei Trennund Und Scheidung
BERATUNGS- UND HILFSANGEBOTE bei Trennung Scheidung in Stadt und StädteRegion Aachen www.trennung-scheidung-aachen.de Sehr geehrte Damen und Herren, Wir freuen uns, Ihnen die aktualisierte Neuauflage der Broschüre „Beratungs- und Hilfsangebote bei Trennung und Scheidung“ des Arbeitskreises Tren- nung/Scheidung Aachen vorstellen zu können. Sie finden in der Broschüre Anlauf- und Beratungs- stellen, bei denen Sie sich während einer Trennung oder Scheidung informieren können und Unterstüt- zung bekommen. Die Broschüre ist in verschiedene Themengebiete (Beratungsstellen allgemein, Hilfe für Alleinerzie- hende, Alleinerziehenden Gruppen in den Kirchen- gemeinden, Gruppen für Kinder in Trennung und Scheidung, Juristische Auskünfte/Rechtsberatung, Psychotherapeutische Hilfen, Meditation) unterteilt. Der erste Teil bezieht sich auf die Stadt Aachen, der zweite Teil auf Beratungsstellen und Hilfsangebote in der StädteRegion Aachen. Für den Arbeitskreis Trennung/Scheidung (Roswitha Damen) Gleichstellungsbeauftragte der Stadt Aachen Gleichstellungsbüro der Stadt Aachen 52058 Aachen Tel: 0241/432-7313 Fax: 0241/4135417999 Email: [email protected] www.aachen.de/gleichstellung Stadt Aachen Beratungsstelle der Arbeiterwohlfahrt (AWO) Gartenstr. 25 52064 Aachen Tel: 0241/889160 Email: [email protected] Diakonisches Werk im Kirchenkreis Aachen e.V. Familien- und Sozialberatung West Vaalser Str. 439 52074 Aachen Tel: 0241/989010 Fax: 0241/9890123 Email: [email protected] Donum Vitae e.V. Schwangerschaftskonfliktsberatung Franzstr. 109 52064 Aachen Tel: 0241/4009977 Fax: 0241/4009888 Email: [email protected] www.aachen.donumvitae.org Fachbereich ElternSchule Aachen – Familienbildung IN VIA Aachen e.V. Krefelder Str. 23 52070 Aachen Tel: 0241/6090815 Fax: 0241/6090820 Email: [email protected] www.elternschule-aachen.de Kath. Erziehungsberatungsstelle der Caritas Reumontstr. -
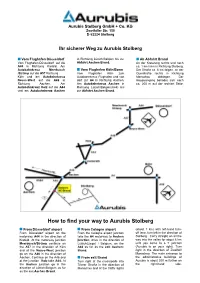
How to Find Your Way to Aurubis Stolberg
Aurubis Stolberg GmbH + Co. KG Zweifaller Str. 150 D-52224 Stolberg Ihr sicherer Weg zu Aurubis Stolberg Vom Flughafen Düsseldorf in Richtung Lüttich/Belgien bis zur Ab Abfahrt Brand Vom Flughafen Düsseldorf auf die Abfahrt Aachen-Brand. An der Kreuzung rechts und nach A44 in Richtung Krefeld. Am ca. 1 km links in Richtung Stolberg. Autobahnkreuz Meerbusch Vom Flughafen Köln/Bonn Der Straße ca. 6 km folgen, an der /Strümp auf die A57 Richtung Vom Flughafen Köln zum Querstraße rechts in Richtung Köln und am Autobahnkreuz Autobahnkreuz Flughafen und von Monschau abbiegen. Der Neuss-West auf die A46 in dort zur A4 in Richtung Aachen. Haupteingang befindet sich nach Richtung Aachen. Am Am Autobahnkreuz Aachen in ca. 200 m auf der rechten Seite. Autobahnkreuz Holz auf die A44 Richtung Lüttich/Belgien(A44) bis und am Autobahnkreuz Aachen zur Abfahrt Aachen-Brand. How to find your way to Aurubis Stolberg From Düsseldorf airport From Cologne airport (about 1 km) with left-hand turn- From Düsseldorf airport on the From the Cologne airport junction off lane, turn left in the direction of motorway A44 in the direction of take the A4 motorway to Aachen Stolberg . Carry straight on all the Krefeld. At the motorway junction junction, drive in the direction of way into the valley for about 6 km Meerbusch/Strümp continue on Lüttich(Liege) / Belgium, on the until you come to a T junction the A57 in the direction of Köln A44 as far as the exit Aachen- (Aurubis is on your right). Turn and at the Neuss-West jonction Brand. -

COMMISSION DECISION of 12 April 2006 Amending Decision 2003/135
13.4.2006EN Official Journal of the European Union L 104/51 COMMISSION DECISION of 12 April 2006 amending Decision 2003/135/EC as regards the extension of plans for the eradication and emergency vaccination of feral pigs against classical swine fever to certain areas of North Rhine- Westfalia and Rhineland-Palatinate and the termination of these plans in other areas of Rhineland- Palatinate (Germany) (notified under document number C(2006) 1531) (Only the German and French texts are authentic) (2006/285/EC) THE COMMISSION OF THE EUROPEAN COMMUNITIES, (3) Germany has also informed the Commission that the classical swine fever situation in certain areas of Rhineland-Palatinate has improved significantly and that Having regard to the Treaty establishing the European the plans for the eradication of classical swine fever and Community, emergency vaccination plans of feral pigs against classical swine fever no longer need to be applied in those areas. (4) Decision 2003/135/EC should therefore be amended Having regard to Council Directive 2001/89/EC of 23 October accordingly. 2001 on Community measures for the control of classical swine fever (1), and in particular Articles 16(1) and 20(2) thereof, (5) The measures provided for in this Decision are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, Whereas: HAS ADOPTED THIS DECISION: Article 1 (1) Commission Decision 2003/135/EC of 27 February 2003 on the approval of the plans for the eradication The Annex to Decision 2003/135/EC is replaced by the text in of classical swine fever and the emergency vaccination of the Annex to this Decision. -

Mining in Central Europe Perspectives from Environmental History
Perspectives Mining in Central Europe Perspectives from Environmental History Edited by FRANK UEKOETTER 2012 / 10 RCC Perspectives Mining in Central Europe Perspectives from Environmental History Edited by Frank Uekoetter 2012 / 10 Mining in Central Europe 3 Contents 05 Introduction: Mining and the Environment Frank Uekoetter 07 Reconstructing the History of Copper and Silver Mining in Schwaz, Tirol Elisabeth Breitenlechner, Marina Hilber, Joachim Lutz, Yvonne Kathrein, Alois Unterkircher, and Klaus Oeggl 21 Mercurial Activity and Subterranean Landscapes: Towards an Environmental History of Mercury Mining in Early Modern Idrija Laura Hollsten 39 Ore Mining in the Sauerland District in Germany: Development of Industrial Mining in a Rural Setting Jan Ludwig 59 Ubiquitous Mining: The Spatial Patterns of Limestone Quarrying in Late Nineteenth-Century Rhineland Sebastian Haumann 71 Uranium Mining and the Environment in East and West Germany Manuel Schramm Mining in Central Europe 5 Frank Uekoetter Introduction: Mining and the Environment The mining exhibit is one of the most acclaimed parts of the Deutsches Museum. It sends visitors on a long, winding underground route that for the most part looks and feels like an actual mine. The exhibit casts a wide net: early modern mining, coal mining, lignite open-pit mining, and mining for ores and salt are all part of the tour, with ore processing and refining making for the grand finale. It is not an exhibit for the claustrophobic, as the path is rather narrow at times, and visitors are expected to complete the entire tour—there are only emergency exits between entrance and finish. However, most visitors enjoy the exhibit, and employees of the Deutsches Museum are accustomed to inquiries as to when they last took the tour. -
EWV-Ladepunkte (Normalladestationen Für AC-Laden)
EWV-Ladepunkte (Normalladestationen für AC-Laden) Die kostenlose eCharge+ App (verfügbar im Apple App oder Google Play Store) bietet einen Ladesäulenfinder und zeigt die Verfügbarkeit der Ladepunkte an. Bei Fragen zum Ladevorgang am Ladepunkt selbst oder zur eCharge+ App, kontaktieren Sie bitte die eMobility Hotline unter: +49 800 22 55 793 LFD Ladestation Ladepunkt Stadt Straße 1 SL00064 BA-1132-8 Aachen Zollernstraße 10 2 SL00064 BA-1153-0 Aachen Zollernstraße 10 3 SL00069 BA-1143-2 Aldenhoven Dietrich-Mühlfahrt-Straße 11-13 4 SL00069 BA-1152-4 Aldenhoven Dietrich-Mühlfahrt-Straße 11-13 5 KP00848 BD-4494-2 Alsdorf Bahnhofstraße 28 6 KP00848 BD-4495-9 Alsdorf Bahnhofstraße 28 7 KP00752 BD-3434-9 Alsdorf Eschweilerstraße 7 8 KP00752 BD-3435-5 Alsdorf Eschweilerstraße 7 9 SL00068 BA-1072-1 Alsdorf Hubertusstraße 17 10 SL00068 BA-1087-1 Alsdorf Hubertusstraße 17 11 KP00858 BD-4573-9 Alsdorf Luisenbad 12 KP00858 BD-4574-5 Alsdorf Luisenbad 13 KP00823 BD-3212-1 Alsdorf Luisenstraße 7 14 KP00823 BD-3213-8 Alsdorf Luisenstraße 7 15 KP00439 BC-3840-0 Alsdorf Theodor-Seipp-Straße 9 16 KP00439 BC-3841-7 Alsdorf Theodor-Seipp-Straße 9 17 KP00312 BC-4226-2 Baesweiler Arnold-Sommerfeld-Ring 211 18 KP00312 BC-4227-9 Baesweiler Arnold-Sommerfeld-Ring 211 19 KP00755 BD-3458-0 Baesweiler Im Bongert 1 20 KP00755 BD-3459-7 Baesweiler Im Bongert 1 21 KP00757 BD-3454-5 Baesweiler Im Sack 3 22 KP00757 BD-3455-1 Baesweiler Im Sack 3 23 SL00187 BA-1378-7 Baesweiler Mariastraße 8 24 SL00187 BA-1379-3 Baesweiler Mariastraße 8 25 KP00756 BD-3456-8 Baesweiler Peterstraße -

M1947 Wiesbaden Central Collecting Point, 1945–1952
M1947 RECORDS CONCERNING THE CENTRAL COLLECTING POINTS (“ARDELIA HALL COLLECTION”): WIESBADEN CENTRAL COLLECTING POINT, 1945–1952 National Archives and Records Administration Washington, DC 2008 United States. National Archives and Records Administration. Records concerning the central collecting points (“Ardelia Hall Collection”) : Wiesbaden Central Collecting Point, 1945–1952.— Washington, D.C. : National Archives and Records Administration, 2008. p. ; cm.-- (National Archives microfilm publications. Publications describing ; M 1947) Cover title. 1. Hall, Ardelia – Archives – Microform catalogs. 2. Germany (Territory under Allied occupation, 1945–1955 : U.S. Zone). Office of Military Government. Property Division – Archives – Microform catalogs. 3. Restitution and indemnification claims (1933– ) – Germany – Microform catalogs. 4. World War, 1939–1945 – Confiscations and contributions – Germany – Archival resources – Microform catalogs. 5. Cultural property – Germany (West) – Archival resources – Microform catalogs. I. Title. INTRODUCTION On 117 rolls of this microfilm publication, M1947, are reproduced the administrative records, photographs of artworks, and property cards from the Wiesbaden Central Collecting Point during the period 1945–52. The Monuments, Fine Arts, and Archives (MFAA) Section recovered Nazi-looted works of art and artifacts from various storage areas and shipped the objects to one of four U.S. central collecting points, including Wiesbaden. In order to research restitution claims, MFAA officers gathered intelligence reports, interrogation reports, captured documents, and general information regarding German art looting. The Wiesbaden records are part of the “Ardelia Hall Collection” in Records of United States Occupation Headquarters, World War II, Record Group (RG) 260. BACKGROUND The basic authority for taking custody of property in Germany was contained in Joint Chiefs of Staff (JCS) Directive 1067/6, which directed the U.S. -

Camp Astrid (A4-Initiative) (No. 081), Stolberg (Rhld.) , Städteregion
EXPOSÉ Camp Astrid (A4-Initiative) Ort: Stolberg (Rhld.) www.germansite.de Regional overview Municipal overview Detail view Parcel Area size 4,500 m² Availability Immediately available area Area designation Commercial zone Divisible No 24h operation No © NRW.Global Business GmbH Page 1 of 4 09/24/2021 EXPOSÉ Camp Astrid (A4-Initiative) Ort: Stolberg (Rhld.) www.germansite.de Details on commercial zone Camp Astrid- perfectly networked properties in an attractive surrounding The area of Camp Astrid comprises ca. 37 hectare. Excluding green und traffic areas, originally ca. 23 hectare were available. Currently ca. 13 hectare are available for selling land in lots. The parceling is flexible from 800 sqm according to individual requirements. An attractive purchasing price of 35.- €/ sqm (fully developed) facilitates the decision for the new location. The location in immediate proximity to Aachen and the advantageous location in the economic area of Europe turn Stolberg into a future-oriented location. Industrial tax multiplier 495.00 % Price 35.00 € / m² Area type GE Links https://www.gistra.de Erreichbarkeit in 20 Minuten: 281.500 Einwohner Transport infrastructure Freeway A 4 5.7 km Freeway A44 7.4 km Airport Maastricht-Aachen 52.3 km Airport Köln-Bonn 68.1 km Port Binnenhavenweg Stein (NL) 48.1 km Port Liège (B) 55.5 km © NRW.Global Business GmbH Page 2 of 4 09/24/2021 EXPOSÉ Camp Astrid (A4-Initiative) Ort: Stolberg (Rhld.) www.germansite.de Information about Stolberg (Rhld.) Stolberg dates back more than 800 years. The town's industrial development began in the 13th century. At the beginning of the 19th century the advent of fine zinc manufacturing and the steam engine hastened the decline of the local lead and zinc industry. -

Wechsel an Der Spitze Der Sparkasse in Eschweiler Und Stolberg
Pressemitteilung Wechsel an der Spitze der Sparkasse in Eschweiler und Stolberg Klaus Wohnaut, Direktor der Sparkasse in Eschweiler und Stolberg, geht zum Monatsende nach 49 Berufsjahren in den wohlverdienten Ruhestand. Sein Nachfolger wird Sascha Schaffrath. Er war seit 2001 das Gesicht der Sparkasse in Eschweiler und in Stolberg: Klaus Wohnaut, der Direktor für die Bereiche Privat- und Geschäftskunden, beendet zum 30. Juni seine Sparkassentätigkeit. Der in Aachen geborene Wohnaut begann 1969 seine Ausbildung bei der damaligen Kreissparkasse Aachen. Nach verschiedenen Einsätzen im Wertpapier- und im Kreditbereich der Sparkasse leitete der Sparkassenbetriebswirt 1982 einige Jahre die Filiale in Broichweiden. 1995 wechselte er in die Firmenkundenbetreuung der Sparkasse und wurde im Jahr 2000 Direktor des Zentralbereichs Privat- und Geschäftskunden in der Aachener Zentrale. Beste Voraussetzungen also, um 2001 die Direktorenposition in Eschweiler und Stolberg zu übernehmen. Hier ist er seitdem verantwortlich für 122 Mitarbeiterinnen und Mitarbeiter in 15 Filialen, in der Vermögensberatung und in den Fachberatungen Wertpapier und Kredit. Für die Kundinnen und Kunden war Klaus Wohnaut immer präsent. Er legte großen Wert darauf, dass alle Kunden bei seiner Sparkasse jederzeit gleich gut und nach ihren jeweiligen Anforderungen und Bedürfnissen beraten werden. In zahlreichen Vereinen war Wohnaut ein gern gesehener Gast und Förderer, lagen ihm die Vereinsarbeit und die Unterstützung des Ehrenamts doch besonders am Herzen. Seite 1 Pressemitteilung Die neu gewonnene Freizeit möchte der Hobby-Tennisspieler und Sportfan Klaus Wohnaut nutzen, um mit seiner Frau oder mit guten Freunden zu verreisen. Aber auch die Kontakte zu den Menschen in Eschweiler und Stolberg möchte der 65-jährige Wohnaut in seinem Ruhestand sofern möglich gerne aufrechthalten. -

042 Änderungssatzung 2 Kfs 01.08.11 1
Bekanntmachung Nr. 042/2011 Satzung vom 13.12.2011 über die Änderung der Satzung der Stadt Herzogenrath über die Inanspruchnahme von Angeboten in der Kindertagespflege und die Erhebung von Elternbeiträgen im Rahmen der Inanspruchnahme von Angeboten in Kindertageseinrichtungen und in Kindertagespflege vom 28.10.2008 -Kinderfördersatzung (Kfs)- in der Fassung der Änderungssatzung vom 18.10.2011 Präambel Der Landesgesetzgeber hat in dem Gesetz zur frühen Bildung und Förderung von Kindern (Kinderbildungsgesetz –KiBiz-) die Betreuung von Kindern in Tageseinrichtungen und in Kindertagespflege unter den Aspekten Erziehung, Bildung, Vereinbarkeit von Familie und Beruf und qualitativer Gleichwertigkeit der Betreuungsangebote landesrechtlich zusammengefasst. Die Jugendämter der Städte Alsdorf, Eschweiler, Herzogenrath, Stolberg und Würselen haben das gemeinsame Ziel, die Förderung von Kindern in Kindertageseinrichtungen und in Kindertagespflege nach einheitlichen Maßstäben abzuwickeln. Dies dient der Rechtssicherheit, Transparenz und Akzeptanz durch die Familien in der Städteregion Aachen. Vor diesem Hintergrund und aufgrund der §§ 7 und 41 der Gemeindeordnung für das Land Nordrhein-Westfalen (GO-NRW) in der Fassung der Bekanntmachung vom 14.07.1994 (GV.NRW. S. 666/SGV. NRW. S. 2023), zuletzt geändert durch Gesetze vom 24.05.2011 (GV. NRW. S. 271) i.V.m. §§ 23, 24, 90 SGB VIII des Achten Buches Sozialgesetzbuch, neugefasst durch Bekanntmachung vom 14.12.2006 (BGBl. I S. 3134), zuletzt geändert durch Art. 3 a des Gesetzes vom 24.03.2011 (BGBl. I S. 453), sowie der §§ 4, 17 und 23 des Gesetzes zur frühen Bildung und Förderung von Kindern (Kinderbildungsgesetz –KiBiz-) vom 30.10.2007 (GV. NRW. S. 462), zuletzt geändert durch Gesetz vom 25.07.2011 (GV. NRW.