Study of Oral Lipid Based Formulation Through Design and Evaluation of Solid Self Emulsified Drug Delivery System International
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

R&D UNICANCER Annual Report 2016
Going further, innovating together R&D UNICANCER Annual Report 2016 Summary Presentation and organisation Regulatory affairs, pharmaco- PAGE 01 vigilance, quality assurance – Ensuring quality and safety 2016 clinical activity in clinical trials. PAGE 08 PAGE 25 Expert groups Epidemiological Strategy and PAGE 16 Medical Economics (ESME) 2016 publications Programme – Harnessing PAGE 21 real-life data in oncology to improve patient care. Clinical operations PAGE 27 PAGE 23 Development Biological Resource and partnerships – Centre (BRC) Optimising collaborations PAGE 24 to foster innovation PAGE 30 Research in the FCCC PAGE 32 Appendices PAGE 33 Contacts, Follow us PAGE 45 GOING FURTHER, INNOVATING TOGETHER R&D ANNUAL REPORT 2016 Presentation and organisation UNICANCER, a major French player in oncology, Acteur majeur de la cancérologie française, groups together 20 French Comprehensive Cancer UNICANCER regroupe les 20 Centres de lutte contre Centers (FCCC). They are private, non-profit health la cancérologie (CLCC), établissements de santé establishments exclusively dedicated to care, research privés à but non lucratif exclusivement dédiés aux and education in cancer. UNICANCER’s R&D soins, à la recherche et à l’enseignement en cancéro- department is the driving force of UNICANCER’s logie. R&D UNICANCER en tant que promoteur research and, as an academic sponsor, it works direc- aca démi que, travaille en direct avec les unités de tly with the research units of the FCCC and other recherche des CLCC et d’autres établissements health establishments (university hospitals, hospitals de santé (CHU, CH, cliniques) en France et à l’inter- and clinics) in France and abroad. The mission of R&D national. -

WO 2018/009638 Al 11 January 2018 (11.01.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/009638 Al 11 January 2018 (11.01.2018) W !P O PCT (51) International Patent Classification: Published: C07D 413/14 (2006.01) A61K 31/553 (2006.01) — with international search report (Art. 21(3)) C07D 403/14 (2006.01) A61P 35/00 (2006.01) — before the expiration of the time limit for amending the (21) International Application Number: claims and to be republished in the event of receipt of PCT/US20 17/040866 amendments (Rule 48.2(h)) (22) International Filing Date: 06 July 2017 (06.07.2017) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/359,001 06 July 2016 (06.07.2016) 62/454,163 03 February 2017 (03.02.2017) (71) Applicant: THE REGENTS OF THE UNIVERSITY OF MICHIGAN [US/US]; Office Of Technology Tran s fer, 1600 Huron Parkway, 2nd Floor, Ann Arbor, MI 48109-2590 (US). (72) Inventors: ROSS, Brian, D.; 2410 Foxway, Ann Arbor, MI 48105 (US). VAN DORT, Marcian; 643 Dornoch Dr., Ann Arbor, MI 48103 (US). (74) Agent: NAPOLI, James, J.; Marshall, Gerstein & Borun LLP, 233 S. Wacker Drive, 6300 Willis Tower, Chicago, IL 60606-6357 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Rediscovery of Fexinidazole
New Drugs against Trypanosomatid Parasites: Rediscovery of Fexinidazole INAUGURALDISSERTATION zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Marcel Kaiser aus Obermumpf, Aargau Basel, 2014 Originaldokument gespeichert auf dem Dokumentenserver der Universität Basel edoc.unibas.ch Dieses Werk ist unter dem Vertrag „Creative Commons Namensnennung-Keine kommerzielle Nutzung-Keine Bearbeitung 3.0 Schweiz“ (CC BY-NC-ND 3.0 CH) lizenziert. Die vollständige Lizenz kann unter creativecommons.org/licenses/by-nc-nd/3.0/ch/ eingesehen werden. 1 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel auf Antrag von Prof. Reto Brun, Prof. Simon Croft Basel, den 10. Dezember 2013 Prof. Dr. Jörg Schibler, Dekan 2 3 Table of Contents Acknowledgement .............................................................................................. 5 Summary ............................................................................................................ 6 Zusammenfassung .............................................................................................. 8 CHAPTER 1: General introduction ................................................................. 10 CHAPTER 2: Fexinidazole - A New Oral Nitroimidazole Drug Candidate Entering Clinical Development for the Treatment of Sleeping Sickness ........ 26 CHAPTER 3: Anti-trypanosomal activity of Fexinidazole – A New Oral Nitroimidazole Drug Candidate for the Treatment -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -
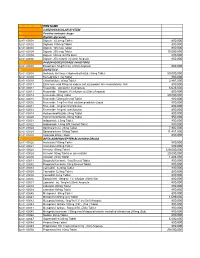
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US). -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Scanned Using Fujitsu 6670 Scanner and Scandall Pro Ver 1.7 Software
1816 1990/826 THE DRUG TARIFF 1990 PURSUANT to section 99 of the Social Security Act 1964, the Minister of Health hereby gives the following direction. ANALYSIS I. Title and commencement 15. Disputes 2. Interpretation PART I PART III GENERAL RULES AFFECTING DIRECTION MISCELLANEOUS PROVISIONS 3. Scope of direction 16. Claims on Department 4. Standards I 7. Allergy treatment sets 5. Charges for requirements 18. Payment for non·disposable syringes and needles PART 11 19. Payment for plastic syringes RULES FOR PRICING 20. Period and quantity of supply for 6. Calculation of payments doctors' and midwives' prescriptions 7. Application of Pricing Schedules 21. Period and quantity of supply for 8. Computation of selling price prescriptions for oral contraceptives 9. Rounding calculations 22. Period and quantity of supply for 10. Minimum charges dentist's prescriptions 11. Adjustment of dose volumes of oral 23. Original packs, and certain antibiotics liquid requirements 24. Bulk supply orders 12. Water 25. Practitioner's supply orders 13. Payment for bulk supply orders 26. Wholesale supply orders 14. Payment for practitioners' supply 27. Revocations and savings orders Schedule DIRECTION 1. Tide and commencement-(I) This direction may be cited as the Drug Tariff 1990. (2) This direction shall come into force on the 1st day of December 1990. 2. Interpretation-(1) In this direction, unless the context otherwise requires,- "The Act" means the Social Security Act 1964: "Board" means an Area Health Board under the Area Health Boards Act 1983 or a Hospital -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Important Note:All Human Products Must Be Of
* Important Note:All human products must be of human recombinant origin wherever these are available in the market *For oral solution it is preferable:Syrup then Suspension and then Elixir MOH CODE ITEM NAME Qty 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1Aa Digtalis glycoside 02-01-00001 digoxin tab 62.5 mcg 800,000 02-01-00002 digitoxin tab 100 mcg 800,000 02-01-00003 digoxin tab 125 mcg 800,000 02-01-00004 digoxin tab 250 mcg 30,415,200 02-01-00005 digoxin PG elixir 50mcg /ml 800,000 02-01-00006 digoxin inj 250 mcg/ml, (2ml amp) 800,000 1Ab PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone inj 5mg/ml (20ml amp) 800,000 1B DIURETICS 02-01-00008 amiloride Hcl 5mg + hydrochlorthiazide 50mg tab 50,000,000 02-01-00009 bumetanide tab 1 mg 1,094,000 02-01-00010 chlorthalidone tab 50mg 3,264,000 02-01-00011 ethacrynic acid as sod.salt inj powder for 800,000 reconstitution 50mg vial 02-01-00012 frusemide inj 20mg/2ml amp 5,780,400 02-01-00013 frusemide I.V. infusion inj 10mg/ml, (25ml amp) 800,000 02-01-00014 frusemide tab 40mg 25,000,000 02-01-00015 frusemide scored tab 500mg 800,000 02-01-00016 frusemide oral solution pead liquid 1mg/1ml 800,000 02-01-00017 frusemide oral solution 4mg/ml 800,000 02-01-00018 frusemide oral solution 8mg/ml 800,000 02-01-00019 hydrochlorothiazide tab 25mg 1,084,000 02-01-00020 hydrochlorothiazide tab 50mg 1,396,000 02-01-00021 indapamide tab 2.5mg 800,000 02-01-00022 Indapamide s/r coated tab 1.5mg 800,000 02-01-00023 spironolactone tab 25mg 5,568,400 02-01-00024 spironolactone tab 100mg 5,147,200 -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data.