Calochortus)From Southern British Columbia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDLT Mountain Home & USFS-Boundry Butte Plant List
CDLT Mountain Home Preserve- Boundary Butte Plant list CDLT Mountain Home & USFS-Boundry Butte Plant list Type Scientific Name Common Name Fern Pteridium aquilinum bracken fern Forb Achillea millefolium common yarrow Forb Agoseris heterophylla annual agoseris Forb Anemone oregana Oregon anemone Forb Antennaria racemosa raceme pussytoes Forb Boechera pauciflorus rockcress (Formerly Arabis) Forb Arnica cordifolia heart-leaf arnica Forb Balsamorhiza sagittata arrowleaf balsamroot Forb Brickellia oblongifolia Mojave brickellbush Forb Cacaliopsis nardosmia silvercrown (Formerly Luina) Forb Calochortus lyallii Lyall's mariposa lily Forb Camassia quamash common camas Forb Castilleja miniata scarlet Indian paintbrush Forb Claytonia lanceolata springbeauty Forb Collinsia parviflora small-flowered blue-eyed mary Forb Commandra umbellata bastard toadflax Forb Delphinium viridescens Wenatchee larkspur Forb Erythronium grandiflorum glacier lily Forb Erysimum species wallflower Forb Fragaria virginiana Virginia strawberry Forb Fritillaria affinis checker lily, chocolate lily Forb Fritillaria pudica yellow bells Forb Galium sp. bedstraw Forb Heuchera cylindrica roundleaf alumroot Forb Hydrophyllum capitatum ballhead waterleaf Forb Lathyrus pauciflorus few-flowered pea Forb Lithophragma parviflorum small-flowered woodland-star Forb Lithophragma glabrum bulbous woodland-star Forb Lithophragma tenellum slender woodland-star Forb Lomatium nudicaule barestem biscuitroot Forb Lomatium triternatum nineleaf biscuitroot Forb Lonicera ciliosa orange honeysuckle -

Squilchuck State Park
Rare Plant Inventory and Community Vegetation Survey Squilchuck State Park Cypripedium montanum,mountain lady’s-slipper, on the state Watch list, present at Squilchuck State Park Conducted for The Washington State Pakrs and Recreation Commission PO Box 42650, Olympia, Washington 98504 Conducted by Dana Visalli, Methow Biodiversity Project PO Box 175, Winthrop, WA 98862 In Cooperation with the Pacific Biodiversity Institute December 31, 2004 Rare Plant Inventory and Community Vegetation Survey Squilchuck State Park In the summer of 2004, at the request of and under contract to the Washington State Parks Commission, a rare plant inventory and community vegetation survey was conducted at Squilchuck State Park by Dana Visalli and assisting botanists and GIS technicians. Squilchuck State Park is a 263 acre park on the east slope of the Cascade Mountains in Central Washington, located largely in the transition zone between shrub-steppe and montane forest. Plant community polygons were delineated prior to the initiation of field surveys using or- thophotos and satellite imagery. These polygons were then ground checked during the vegetation surveys, which were conducted simultaneously with the rare plant inventories. All plant associa- tions were determined using theField Guide for Forested Plant Associations of the Wenatchee National Forest(Lilybridge et al, 1995) The Douglas-fir dominated forest above the lodge, on the eastern slopes of the park. The forest on this east slope is in places heavily overstocked and the trees supressed. Vegetation surveys and plant inventories were conducted by two field personnel (one bota- nist, one GIS technician) on June 11th, and again by 4 field workers on August 13 (two botanists and two GIS technicians). -

Habitat Use of Black Bears in the Northeast Cascades of Washington
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1997 Habitat use of black bears in the northeast Cascades of Washington Andrea Lee. Gold The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Gold, Andrea Lee., "Habitat use of black bears in the northeast Cascades of Washington" (1997). Graduate Student Theses, Dissertations, & Professional Papers. 6411. https://scholarworks.umt.edu/etd/6411 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Maureen and Mike MANSFIELD LIBRARY The Universityof IVIONTANA Permission is granted by tlie autlior to reproduce tliis material in its entirety, provided tliat tliis material is used for scholarly purposes and is properly cited in published works and reports. ** ** Please check "Yes" or "No" and provide signature Yes, I grant pennission No, I do not grant permission Author's Signature Date Æ u j. Any copying for commercial purposes or financial gain may be undertaken only with the author's explicit consent. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. \ f HABITAT USE OF BLACK BEARS IN THE NORTHEAST CASCADES OF WASHINGTON By Andrea Lee Gold B A , The University of San Diego, 1993 Presented in partial fulfillment of the requirements for the degree of Master of Science THE UNIVERSITY OF MONTANA 1997 Approved W: / / y Chairman, Board of Exagiiners Dean, Graduate School q -9 -? 7 Date Reproduced with permission of the copyright owner. -

Lyall's Mariposa Lily (Calochortus Lyallii)
PROPOSED Species at Risk Act Management Plan Series Adopted under Section 69 of SARA Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in Canada Lyall’s Mariposa Lily 2017 Recommended citation: Environment and Climate Change Canada. 2017. Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in Canada [Proposed]. Species at Risk Act Management Plan Series. Environment and Climate Change Canada, Ottawa. 2 parts, 3 pp. + 19 pp. For copies of the management plan, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: Kella Sadler, Environment and Climate Change Canada Également disponible en français sous le titre « Plan de gestion du calochorte de Lyall (Calochortus lyallii) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change, 2017. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 MANAGEMENT PLAN FOR THE LYALL’S MARIPOSA LILY (Calochortus lyallii) IN CANADA 2017 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of British Columbia has given permission to the Government of Canada to adopt the Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in British Columbia (Part 2) under section 69 of the Species at Risk Act (SARA). -

Lyall's Mariposa Lily (Calochortus Lyallii)
COSEWIC Assessment and Status Report on the Lyall’s Mariposa Lily Calochortus lyallii in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Lyall’s Mariposa Lily Calochortus lyallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 34 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2001. COSEWIC assessment and status report on the the Lyall’s Mariposa Lily Calochortus lyallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 24 pp. M.T. Miller and G.W. Douglas. 2001. COSEWIC status report on the Lyall’s Mariposa Lily Calochortus lyallii in Canada, in COSEWIC assessment and status report on the Lyall’s Mariposa Lily Calochortus lyallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-24 pp. Production note: COSEWIC would like to acknowledge Michael Miller for writing the status report on Lyall’s Mariposa Lily Calochortus lyallii in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Erich Haber, Bruce Bennett, and Jeannette Whitton, COSEWIC Vascular Plants Specialist Subcommittee Co-chairs. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la calochorte de Lyall (Calochortus lyallii) au Canada. -
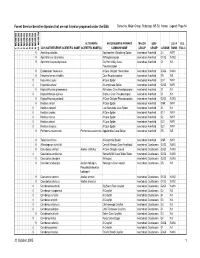
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

A Checklist of Vascular Plants Endemic to California
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 3-2020 A Checklist of Vascular Plants Endemic to California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "A Checklist of Vascular Plants Endemic to California" (2020). Botanical Studies. 42. https://digitalcommons.humboldt.edu/botany_jps/42 This Flora of California is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A LIST OF THE VASCULAR PLANTS ENDEMIC TO CALIFORNIA Compiled By James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California 13 February 2020 CONTENTS Willis Jepson (1923-1925) recognized that the assemblage of plants that characterized our flora excludes the desert province of southwest California Introduction. 1 and extends beyond its political boundaries to include An Overview. 2 southwestern Oregon, a small portion of western Endemic Genera . 2 Nevada, and the northern portion of Baja California, Almost Endemic Genera . 3 Mexico. This expanded region became known as the California Floristic Province (CFP). Keep in mind that List of Endemic Plants . 4 not all plants endemic to California lie within the CFP Plants Endemic to a Single County or Island 24 and others that are endemic to the CFP are not County and Channel Island Abbreviations . -

10.0 References Cited
10.0 REFERENCES CITED Aars, J., and R.A. Ims, "The Effect of Habitat Corridors on Rates of Transfer and Interbreeding Between Vole Demes" (1999). Abrams, L., "Illustrated Flora of the Pacific States" (1923). Adams, T.E., et al., "Blue and Valley Oak Seedling Establishment on California's Hardwood Rangelands" (1991). Advisory Council on Historic Preservation, "Section 106 Archaeology Guidance" (2003). AirPhotoUSA, "200- and 400-Scale False-Color Digital Orthographic Maps" (2007). Akenson, J., et al., "Diurnal Bedding Habitat of Mountain Lions in Northeast Oregon" (1996). Albrecht, D.J., and L.W. Oring, "Song in Chipping Sparrows, Spizella passerina, Structure and Function" (1995). Alcorn, J.R., "The Birds of Nevada" (1988). Aldrich, E.C., "Nesting of the Allen Hummingbird" (1945). Allaire, P.N., and C.D. Fisher, "Feeding Ecology of Three Resident Sympatric Sparrows in Eastern Texas" (1975). Allen, E., "Characterizing the Habitat of Slender-Horned Spineflower (Dodecahema leptoceras), prepared for the California Department of Fish and Game" (1996). Allen, E.B., et al., "Nitrogen-Deposition Effects on Coastal Sage Vegetation of Southern California" (1996). Allen-Diaz, B., et al., "Oak Woodlands and Forests" (2007). Allan E. Seward Engineering Geology, Inc., "Addendum No. 1 Preliminary Geologic Report for Newhall Ranch" (December 4, 1995). "Addendum No. 2, Response to County Comments for Newhall Ranch Specific Plan" (May 13, 1996). "Preliminary Geologic Feasibility Report for Offsite Extensions of Magic Mountain Parkway and Valencia Boulevard to Newhall Ranch" (December 13, 1995). "Preliminary Geologic Report for Newhall Ranch" (September 19, 1994). Ambrose, R.F., et al., "An Evaluation of Compensatory Mitigation Projects Permitted Under Clean Water Act Section 401 by the California State Water Quality Control Board, 1991–2002, prepared for the California State Water Resources Control Board" (August 2006). -

Lyall's Mariposa Lily (Calochortus Lyallii)
COSEWIC Assessment and Status Report on the Lyall’s Mariposa Lily Calochortus lyallii in Canada THREATENED 2001 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION DES ENDANGERED WILDLIFE ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: Please note: Persons wishing to cite data in the report should refer to the report (and cite the author(s)); persons wishing to cite the COSEWIC status will refer to the assessment (and cite COSEWIC). A production note will be provided if additional information on the status report history is required. COSEWIC 2001. COSEWIC assessment and status report on the the Lyall’s mariposa lily Calochortus lyallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 24 pp. M.T. Miller and G.W. Douglas. 2001. COSEWIC status report on the Lyall’s mariposa lily Calochortus lyallii in Canada, in COSEWIC assessment and status report on the Lyall’s mariposa lily Calochortus lyallii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-24 pp. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la calochorte de Lyall (Calochortus lyallii) au Canada Cover illustration: Lyall’s mariposa lily — Line drawing by Jane Lee Ling in Douglas et al. -

Management Plan for the Lyall's Mariposa Lily (Calochortus Lyallii)
Species at Risk Act Management Plan Series Adopted under Section 69 of SARA Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in Canada Lyall’s Mariposa Lily 2018 Recommended citation: Environment and Climate Change Canada. 2018. Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in Canada. Species at Risk Act Management Plan Series. Environment and Climate Change Canada, Ottawa. 2 parts, 3 pp. + 19 pp. For copies of the management plan, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: Kella Sadler, Environment and Climate Change Canada Également disponible en français sous le titre « Plan de gestion du calochorte de Lyall (Calochortus lyallii) au Canada » © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change, 2018. All rights reserved. ISBN 978-0-660-24354-2 Catalogue no. En3-5/90-2018E-PDF Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 MANAGEMENT PLAN FOR THE LYALL’S MARIPOSA LILY (Calochortus lyallii) IN CANADA 2018 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of British Columbia has given permission to the Government of Canada to adopt the Management Plan for the Lyall’s Mariposa Lily (Calochortus lyallii) in British Columbia (Part 2) under section 69 of the Species at Risk Act (SARA). -

Eastern Washington Plant List
The NatureMapping Program Revised: 9/15/2011 Eastern Washington Plant List - Scientific Name 1- Non- native, 2- ID Scientific Name Common Name Plant Family Invasive √ 1141 Abies amabilis Pacific silver fir Pinaceae 1 Abies grandis Grand fir Pinaceae 1142 Abies lasiocarpa Sub-alpine fir Pinaceae 762 Abronia mellifera White sand verbena Nyctaginaceae 1143 Abronia umbellata Pink sandverbena Nyctaginaceae 763 Acer glabrum Douglas maple Aceraceae 3 Acer macrophyllum Big-leaf maple Aceraceae 470 Acer platinoides* Norway maple Aceraceae 1 5 Achillea millifolium Yarrow Asteraceae 1144 Aconitum columbianum Monkshood Ranunculaceae 8 Actaea rubra Baneberry Ranunculaceae 9 Adenocaulon bicolor Pathfinder Asteraceae 10 Adiantum pedatum Maidenhair fern Polypodiaceae 764 Agastache urticifolia Nettle-leaf horse-mint Lamiaceae 1145 Agoseris aurantiaca Orange agoseris Asteraceae 1146 Agoseris elata Tall agoseris Asteraceae 705 Agoseris glauca Mountain agoseris Asteraceae 608 Agoseris grandiflora Large-flowered agoseris Asteraceae 716 Agoseris heterophylla Annual agoseris Asteraceae 11 Agropyron caninum Bearded wheatgrass Poaceae 560 Agropyron cristatum* Crested wheatgrass Poaceae 1 1147 Agropyron dasytachyum Thickspike wheatgrass Poaceae 739 Agropyron intermedium* Intermediate ryegrass Poaceae 1 12 Agropyron repens* Quack grass Poaceae 1 744 Agropyron smithii Bluestem Poaceae 523 Agropyron spicatum Blue-bunch wheatgrass Poaceae 687 Agropyron trachycaulum Slender wheatgrass Poaceae 13 Agrostis alba* Red top Poaceae 1 799 Agrostis exarata* Spike bentgrass -

Appendix 6.8-A
Appendix 6.8-A Terrestrial Wildlife and Vegetation Baseline Report AJAX PROJECT Environmental Assessment Certificate Application / Environmental Impact Statement for a Comprehensive Study Ajax Mine Terrestrial Wildlife and Vegetation Baseline Report Prepared for KGHM Ajax Mining Inc. Prepared by This image cannot currently be displayed. Keystone Wildlife Research Ltd. #112, 9547 152 St. Surrey, BC V3R 5Y5 July 2015 Ajax Mine Terrestrial Wildlife and Vegetation Baseline Keystone Wildlife Research Ltd. DISCLAIMER This report was prepared exclusively for KGHM Ajax Mining Inc. by Keystone Wildlife Research Ltd. The quality of information, conclusions and estimates contained herein is consistent with the level of effort expended and is based on: i) information available at the time of preparation; ii) data collected by Keystone Wildlife Research Ltd. and/or supplied by outside sources; and iii) the assumptions, conditions and qualifications set forth in this report. This report is intended for use by KGHM Ajax Mining Inc. only, subject to the terms and conditions of its contract with Keystone Wildlife Research Ltd. Any other use, or reliance on this report by any third party, is at that party’s sole risk. 2 Ajax Mine Terrestrial Wildlife and Vegetation Baseline Keystone Wildlife Research Ltd. EXECUTIVE SUMMARY Baseline wildlife and habitat surveys were initiated in 2007 to support a future impact assessment for the redevelopment of two existing, but currently inactive, open pit mines southwest of Kamloops. Detailed Project plans were not available at that time, so the general areas of activity were buffered to define a study area. The two general Project areas at the time were New Afton, an open pit just south of Highway 1, and Ajax, east of Jacko Lake.