Neuropharmacological Studies of Piper Auritum Kunth (Piperaceae): Antinociceptive and Anxiolytic-Like Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

62 of 17 January 2018 Replacing Annex I to Regulation (EC) No 396/2005 of the European Parliament and of the Council
23.1.2018 EN Official Journal of the European Union L 18/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) 2018/62 of 17 January 2018 replacing Annex I to Regulation (EC) No 396/2005 of the European Parliament and of the Council (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (1), and in particular Article 4 thereof, Whereas: (1) The products of plant and animal origin to which the maximum residue levels of pesticides (‘MRLs’) set by Regulation (EC) No 396/2005 apply, subject to the provisions of that Regulation, are listed in Annex I to that Regulation. (2) Additional information should be provided by Annex I to Regulation (EC) No 396/2005 as regards the products concerned, in particular as regards the synonyms used to indicate the products, the scientific names of the species to which the products belong and the part of the product to which the respective MRLs apply. (3) The text of footnote (1) in both Part A and Part B of Annex I to Regulation (EC) No 396/2005 should be reworded, in order to avoid ambiguity and different interpretations encountered with the current wording. (4) New footnotes (3) and (4) should be inserted in Part A of Annex I to Regulation (EC) No 396/2005, in order to provide additional information as regards the part of the product to which the MRLs of the products concerned apply (5) New footnote (7) should be inserted in Part A of Annex I to Regulation (EC) No 396/2005, in order to clarify that MRLs of honey are not applicable to other apiculture products due to their different chemicals character istics. -

The Igbo Traditional Food System Documented in Four States in Southern Nigeria
Chapter 12 The Igbo traditional food system documented in four states in southern Nigeria . ELIZABETH C. OKEKE, PH.D.1 . HENRIETTA N. ENE-OBONG, PH.D.1 . ANTHONIA O. UZUEGBUNAM, PH.D.2 . ALFRED OZIOKO3,4. SIMON I. UMEH5 . NNAEMEKA CHUKWUONE6 Indigenous Peoples’ food systems 251 Study Area Igboland Area States Ohiya/Ohuhu in Abia State Ubulu-Uku/Alumu in Delta State Lagos Nigeria Figure 12.1 Ezinifite/Aku in Anambra State Ede-Oballa/Ukehe IGBO TERRITORY in Enugu State Participating Communities Data from ESRI Global GIS, 2006. Walter Hitschfield Geographic Information Centre, McGill University Library. 1 Department of 3 Home Science, Bioresources Development 5 Nutrition and Dietetics, and Conservation Department of University of Nigeria, Program, UNN, Crop Science, UNN, Nsukka (UNN), Nigeria Nigeria Nigeria 4 6 2 International Centre Centre for Rural Social Science Unit, School for Ethnomedicine and Development and of General Studies, UNN, Drug Discovery, Cooperatives, UNN, Nigeria Nsukka, Nigeria Nigeria Photographic section >> XXXVI 252 Indigenous Peoples’ food systems | Igbo “Ndi mba ozo na-azu na-anwu n’aguu.” “People who depend on foreign food eventually die of hunger.” Igbo saying Abstract Introduction Traditional food systems play significant roles in maintaining the well-being and health of Indigenous Peoples. Yet, evidence Overall description of research area abounds showing that the traditional food base and knowledge of Indigenous Peoples are being eroded. This has resulted in the use of fewer species, decreased dietary diversity due wo communities were randomly to household food insecurity and consequently poor health sampled in each of four states: status. A documentation of the traditional food system of the Igbo culture area of Nigeria included food uses, nutritional Ohiya/Ohuhu in Abia State, value and contribution to nutrient intake, and was conducted Ezinifite/Aku in Anambra State, in four randomly selected states in which the Igbo reside. -

Edible Leafy Plants from Mexico As Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: a Review
antioxidants Review Edible Leafy Plants from Mexico as Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: A Review Lourdes Mateos-Maces 1, José Luis Chávez-Servia 2,* , Araceli Minerva Vera-Guzmán 2 , Elia Nora Aquino-Bolaños 3 , Jimena E. Alba-Jiménez 4 and Bethsabe Belem Villagómez-González 2 1 Recursos Genéticos y Productividad-Genética, Colegio de Posgraduados, Carr. México-Texcoco Km. 36.5, Montecillo, Texcoco 56230, Mexico; [email protected] 2 CIIDIR-Oaxaca, Instituto Politécnico Nacional, Ciudad de México 07738, Mexico; [email protected] (A.M.V.-G.); [email protected] (B.B.V.-G.) 3 Centro de Investigación y Desarrollo de Alimentos, Universidad Veracruzana, Xalapa-Enríquez 1090, Mexico; [email protected] 4 CONACyT-Centro de Investigación y Desarrollo de Alimentos, Universidad Veracruzana, Xalapa-Enríquez 1090, Mexico; [email protected] * Correspondence: [email protected] Received: 15 May 2020; Accepted: 13 June 2020; Published: 20 June 2020 Abstract: A review of indigenous Mexican plants with edible stems and leaves and their nutritional and nutraceutical potential was conducted, complemented by the authors’ experiences. In Mexico, more than 250 species with edible stems, leaves, vines and flowers, known as “quelites,” are collected or are cultivated and consumed. The assessment of the quelite composition depends on the chemical characteristics of the compounds being evaluated; the protein quality is a direct function of the amino acid content, which is evaluated by high-performance liquid chromatography (HPLC), and the contribution of minerals is evaluated by atomic absorption spectrometry, inductively coupled plasma-optical emission spectrometry (ICP-OES) or ICP mass spectrometry. The total contents of phenols, flavonoids, carotenoids, saponins and other general compounds have been analyzed using UV-vis spectrophotometry and by HPLC. -
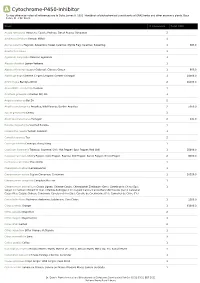
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Antimicrobial Activity of Five Plant Extracts and Synthetic Fungicide in the Management of Postharvest Pathogens of Yam (Dioscorea Rotundata Poir) in Storage
Open Access Journal of Microbiology & Biotechnology ISSN: 2576-7771 Antimicrobial Activity of Five Plant Extracts and Synthetic Fungicide in the Management of Postharvest Pathogens of Yam (Dioscorea Rotundata Poir) in Storage Gwa VI1,2*, Nwankiti AO1 and Hamzat OTH3 Research Article 1 Department of Crop and Environmental Protection, Federal University of Agriculture, Volume 3 Issue 1 Nigeria Received Date: July 07, 2018 2Department of Crop Production and Protection, Federal University, Nigeria Published Date: August 17, 2018 DOI: 10.23880/oajmb-16000128 3Crop Research Program, Federal University of Agriculture, Nigeria *Corresponding author: Gwa VI, Department of Crop and Environmental Protection, Federal University of Agriculture, PM B 2373 Makurdi, Nigeria, Email: [email protected] Abstract Crude extracts leaves of Azadirachta indica A. Juss. (neem), Nicotiana tabacum Linn. (Tobacco), rhizomes of Zingiber officinale Rosc. (Ginger), leaves of Carica papaya Lam. (pawpaw) and seeds of Piper guineense Schumach. (Black pepper) were tested at 30 g/L, 60 g/L and 90 g/L as well as mancozeb at 4 g/L, 8 g/L and 12 g/L concentrations against Aspergillus ochraceus in vitro. Rotted yam tubers were collected from farmers’ barns forth nightly for four times. Treatments were replicated three times and completely randomized. Data were subjected to analysis of variance (ANOVA) and means were separated using Fisher’s least significance difference (LSD). Mycelial growth of A. ochraceus was inhibited by these extracts. Percentage growth inhibitions at 30 g/L ranged from 40.19% (N. tabacum) to 51.95% (P. guineense) while percentage growth inhibitions at 60 g/L ranged between 57.47% (N. -

Life Science Journal 2013; 10(4) 3411
Life Science Journal 2013; 10(4) http://www.lifesciencesite.com Aflatoxin and Ochratoxin A residues in some meat additives Waleed Rizk El-Ghreeb, Wageh Sobhy Darwish, Ahmed Elsayed Tharwat, Kamal Ibrahim EL-Desoky and Mohamed Abdallah Hussein* Food Control Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, 44519 Egypt Email: [email protected] * Abstract: Contamination of meat additives with mould spores and mycotoxins like, aflatoxin and ochratoxin, is a major problem in many developing countries like Egypt as it leads to great public health hazards if consumed directly or added to the meat products. In this study, we investigated the contamination of some meat additives (Starch, soy flour, nutmeg, cumin, black pepper and red pepper) with different mould genera and subsequently screened their contamination potential with some mycotoxins like aflatoxin and ochratoxin A. Our results declared that, 100 % of the meat additive and spices were contaminated by mould. Aspergillus was the most predominant genus, (detected in 100% of samples), which identified into six species. Aspergillus niger and Aspergillus flavus were the most common Aspergillus species in this study. The means of aflatoxin residues in examined meat additive and spices arranged in descending manner as following: nutmeg > red pepper > soy-flour > starch > cummin > black pepper. However, ochratoxin A residues in examined meat additive and spices was ordered as following soy flour > nutmeg > starch > red pepper > cummin > black pepper. Great care should be taken during selection of the meat additives before introducing them to food commodities. [Waleed Rizk El-Ghreeb, Wageh Sobhy Darwish, Ahmed Elsayed Tharwat, Kamal Ibrahim EL-Desoky and Mohamed Abdallah Hussein. -
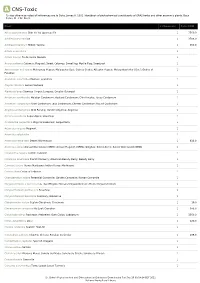
Show Activity
A CNS-Toxic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Achillea moschata Iva 1 3708.0 Achillea millefolium Milfoil; Yarrow 1 550.0 Acinos suaveolens 1 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Calamus; Flagroot; Sweet Calamus; Sweetflag; Myrtle Flag; Sweetroot 1 Aframomum melegueta Melegueta Pepper; Malagueta (Sp.); Guinea Grains; Alligator Pepper; Malagettapfeffer (Ger.); Grains-of- 1 Paradise Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 Amomum xanthioides Malabar Cardamom; Bastard Cardamom; Chin Kousha; Tavoy Cardamom 1 Amomum compactum Siam Cardamom; Java Cardamom; Chester Cardamom; Round Cardamom 1 Angelica archangelica Wild Parsnip; Garden Angelica; Angelica 1 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Virginia Snakeroot; Serpentaria 1 Artemisia vulgaris Mugwort 1 Artemisia salsoloides 1 Artemisia herba-alba Desert Wormwood 1 638.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Qinghao; Sweet Annie; Sweet Wormwood (GRIN) 1 Calamintha nepeta Turkish Calamint 1 Callicarpa americana French Mulberry; American Beauty Berry; Beauty Berry 1 Cannabis sativa Hemp; Marijuana; Indian Hemp; Marihuana 1 Cedrus libani Cedar of Lebanon 1 Chamaemelum nobile Perennial Camomile; Garden Camomile; Roman Camomile -

Spice Large.Pdf
Gernot Katzer’s Spice List (http://gernot-katzers-spice-pages.com/engl/) 1/70 (November 2015) Important notice Copyright issues This document is a byproduct of my WWW spice pages. It lists names of spices in about 100 different languages as well as the sci- This document, whether printed or in machine-readable form, may entific names used by botanists and pharmacists, and gives for each be copied and distributed without charge, provided the above no- local name the language where it is taken from and the botanical tice and my address are retained. If the file content (not the layout) name. This index does not tell you whether the plant in question is is modified, this should be indicated in the header. discussed extensively or is just treated as a side-note in the context of another spice article. Employees of Microsoft Corporation are excluded from the Another point to make perfectly clear is that although I give my above paragraph. On all employees of Microsoft Corporation, a best to present only reliable information here, I can take no warrant licence charge of US$ 50 per copy for copying or distributing this of any kind that this file, or the list as printed, or my whole WEB file in all possible forms is levied. Failure to pay this licence charge pages or anything else of my spice collection are correct, harm- is liable to juristical prosecution; please contact me personally for less, acceptable for non-adults or suitable for any specific purpose. details and mode of paying. All other usage restrictions and dis- Remember: Anything free comes without guarantee! claimers decribed here apply unchanged. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

Report to the Government of Samoa on Invasive Plant Species of Environmental Concern
Report to the Government of Samoa on Invasive Plant Species of Environmental Concern James C. Space and Tim Flynn U.S.D.A. Forest Service Pacific Southwest Research Station Institute of Pacific Islands Forestry Honolulu, Hawai‘i, USA 26 November 2002 Table of Contents Report to the Government of Samoa on Invasive Plant Species of Environmental Concern................................................1 1. Dangerous species not known to be in Samoa..................................................................................................................2 2. Species that are invasive or have the potential to become so in Samoa..........................................................................5 Invasive species already widespread in Samoa ................................................................................................................5 Invasive species of limited extent......................................................................................................................................8 3. Species that are known or listed as weedy or invasive elsewhere and are common, weedy or cultivated in Samoa ....................................................................................................................................................................................11 4. Native species (or Polynesian introductions) exhibiting aggressive behavior..............................................................13 Strategies for dealing with invasive species........................................................................................................................13 -

ROOT BEER PLANT Or MEXICAN PEPPERLEAF Piper Auritum
ROOT BEER PLANT or MEXICAN PEPPERLEAF Piper auritum Characteristics Perennial Spread: 2’ – 12’ Zone: 8 to 12 Leaves: Large, Fragrant, heart-shaped Sun: Partial or dappled shade Large bush or small tree Height: 2’ – 12’ Culture A root beer plant growing in the garden provides an interesting fragrance. A root beer plant, also known as Hoja Santa, holy leaf or Mexican pepperleaf, growing in the garden provides the aroma of root beer, and large, furry leaves in which to wrap foods and give them a hint of root beer flavor. An evergreen shrub or small tree in USDA zones 10 and 11, root beer plants are herbaceous perennials in USDA zones 8 and 9. Flowers of the root beer plant are not showy and sometimes not even noticeable. Plant it in full sun to part shade, feed and water occasionally. Caring for root beer plants can be neglected without the loss of the plant, but the most attractive foliage results from proper care. The plant won’t survive in freezing temperatures. Noteworthy Characteristics Root beer plants are primarily used as culinary ingredients, or in some areas, medicinal. Native to Mexico, this plant has a diversity of uses. Leaves of the root beer plant are steamed and used as wraps in many native dishes. The leaves may also be chopped for use in cooking or salads. Info about root beer plants says they are also used medicinally as an aid to digestion and to calm colicky babies. Other info says it is used for bronchitis and asthma. However, in the United States, the FDA banned its commercial use as root beer flavoring in the 1960’s, as it contains the oil safrole, which is known to be carcinogenic in animals. -

Herb List Gardens
NORTH HAVEN Herb List Gardens Common Name Botanical Name UsesCat Light Color Height Soil Symbolism ALOE VERA Aloe barbadensis M TT SUNOrangeWD12"-18" Healing ANISE Pimpinella anisum CMTA S/PSH White12"-18" MWD APPLE MINT Mentha suaveolens CFr MTP S/PSHWhite12"-18" MWD Virtue APPLE MINT Mentha rotundifolia S/PSH Mauve4"-6" M ARUGULA Eruca vesicara CM A SUNCream18"-24" MWD Enthusiasm ARUGULA, DWARF Diplotaxis erucoides C A SUNYellow10"-12" WD Straightforward BASIL, AFRICAN BLUE Ocimum kilimandscharicum CFr O A SUNPurple24"-36" M Affection BASIL, AROMA 2 Ocimum basilicum CFr Fl M O A S/PSHWhite18"-24" WD Good Luck BASIL,' AUSSIE SWEETIE' Ocimum basilicum CFr A SUN18"-24" WD Good Wishes BASIL, BOXWOOD Ocimum basilicum CFr Fl O A S/PSH White8"-10" WD BASIL, CINNAMON Ocimum basilicum CFr A SUNLavender 18"-24" MWD Good Wishes BASIL, 'CITRIODORUM' LEMON Ocimum basilicum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, DARK OPAL Ocimum basilicum COA SUNPurple18"-24" MWD Good Wishes BASIL, 'GENOVESE' Ocimum basilicum CFr Fl A S/PSHWhite18"-24" MWD Good Wishes BASIL, 'GREEK COLUMNAR' OR 'AU Ocimum xcitriodorum 'Lesbos' CFr MO A S/PSH 24"-36" MWD BASIL, HOLY Ocimum sanctum Fr Fl A S/PSHWhite or Laven18"-24" MWD Good Luck BASIL, LETTUCE LEAF Ocimum basilicum COA SUNWhite18"-24" MWD Good Wishes BASIL, LIME Ocimum americanum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, 'MAGICAL MICHAEL' Ocimum basilicum CFr Fl O A S/PSHWhite18"-24" WD Good Wishes BASIL, 'MINETTE' Ocimum basilicum CFr O A SUNWhite12"-18" MWD Good Wishes BASIL, MINI PURPLE Ocimum basilicum