Saline Hydrides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electronic Structure and Crystal Phase Stability of Palladium Hydrides
Electronic structure and crystal phase stability of palladium hydrides Abdesalem Houari∗ Theoretical Physics Laboratory, Department of Physics, University of Bejaia, Bejaia, Algeria Samir F. Matar† CNRS, ICMCB, Universit´ede Bordeaux, 33600 Pessac, France Volker Eyert‡ Materials Design SARL, 92120 Montrouge, France (Dated: November 4, 2014) The results of electronic structure calculations for a variety of palladium hydrides are presented. The calculations are based on density functional theory and used different local and semilocal approximations. The thermodynamic stability of all structures as well as the electronic and chemical bonding properties are addressed. For the monohydride, taking into account the zero-point energy is important to identify the octahedral Pd-H arrangement with its larger voids and, hence, softer hydrogen vibrational modes as favorable over the tetrahedral arrangement as found in the zincblende and wurtzite structures. Stabilization of the rocksalt structure is due to strong bonding of the 4d and 1s orbitals, which form a characteristic split-off band separated from the main d-band group. Increased filling of the formerly pure d states of the metal causes strong reduction of the density of states at the Fermi energy, which undermines possible long-range ferromagnetic order otherwise favored by strong magnetovolume effects. For the dihydride, octahedral Pd-H arrangement as realized e.g. in the pyrite structure turns out to be unstable against tetrahedral arrangemnt as found in the fluorite structure. Yet, from both heat of formation and chemical bonding considerations the dihydride turns out to be less favorable than the monohydride. Finally, the vacancy ordered defect phase Pd3H4 follows the general trend of favoring the octahedral arrangement of the rocksalt structure for Pd:H ratios less or equal to one. -
Chapter 13.Pptx
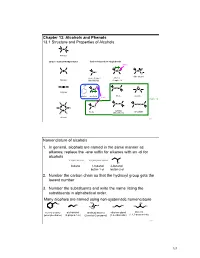
Chapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic O C C C N C N C X O nitro alkane X= F, Cl, Br, I amines Alkenes Alkyl Halide Chapter 23 OH C C H O C O C C O C C Alkynes phenol alcohols ethers epoxide acidic Chapter 14 H H H C S C C C C S S C C S C C H C C sulfides thiols disulfide H H (thioethers) Arenes 253 Nomenclature of alcohols 1. In general, alcohols are named in the same manner as alkanes; replace the -ane suffix for alkanes with an -ol for alcohols CH3CH2CH2CH3 CH3CH2CH2CH2OH OH butane 1-butanol 2-butanol butan-1-ol butan-2-ol 2. Number the carbon chain so that the hydroxyl group gets the lowest number 3. Number the substituents and write the name listing the substituents in alphabetical order. Many alcohols are named using non-systematic nomenclature H C OH 3 OH OH C OH OH HO OH H3C HO H3C benzyl alcohol allyl alcohol tert-butyl alcohol ethylene glycol glycerol (phenylmethanol) (2-propen-1-ol) (2-methyl-2-propanol) (1,2-ethanediol) (1,2,3-propanetriol) 254 127 Alcohols are classified according to the H R C OH C OH H H degree of substitution of the carbon bearing H H 1° carbon the -OH group methanol primary alcohol primary (1°) : one alkyl substituent R R C OH C OH R R secondary (2°) : two alkyl substituents H R 2° carbon 3° carbon tertiary (3°) : three alkyl substituents secondary alcohol tertiary alcohol Physical properties of alcohols – the C-OH bond of alcohols has a significant dipole moment. -

Radical Approaches to Alangium and Mitragyna Alkaloids
Radical Approaches to Alangium and Mitragyna Alkaloids A Thesis Submitted for a PhD University of York Department of Chemistry 2010 Matthew James Palframan Abstract The work presented in this thesis has focused on the development of novel and concise syntheses of Alangium and Mitragyna alkaloids, and especial approaches towards (±)-protoemetinol (a), which is a key precursor of a range of Alangium alkaloids such as psychotrine (b) and deoxytubulosine (c). The approaches include the use of a key radical cyclisation to form the tri-cyclic core. O O O N N N O O O H H H H H H O N NH N Protoemetinol OH HO a Psychotrine Deoxytubulosine b c Chapter 1 gives a general overview of radical chemistry and it focuses on the application of radical intermolecular and intramolecular reactions in synthesis. Consideration is given to the mediator of radical reactions from the classic organotin reagents, to more recently developed alternative hydrides. An overview of previous synthetic approaches to a range of Alangium and Mitragyna alkaloids is then explored. Chapter 2 follows on from previous work within our group, involving the use of phosphorus hydride radical addition reactions, to alkenes or dienes, followed by a subsequent Horner-Wadsworth-Emmons reaction. It was expected that the tri-cyclic core of the Alangium alkaloids could be prepared by cyclisation of a 1,7-diene, using a phosphorus hydride to afford the phosphonate or phosphonothioate, however this approach was unsuccessful and it highlighted some limitations of the methodology. Chapter 3 explores the radical and ionic chemistry of a range of silanes. -

Reductions and Reducing Agents
REDUCTIONS AND REDUCING AGENTS 1 Reductions and Reducing Agents • Basic definition of reduction: Addition of hydrogen or removal of oxygen • Addition of electrons 9:45 AM 2 Reducible Functional Groups 9:45 AM 3 Categories of Common Reducing Agents 9:45 AM 4 Relative Reactivity of Nucleophiles at the Reducible Functional Groups In the absence of any secondary interactions, the carbonyl compounds exhibit the following order of reactivity at the carbonyl This order may however be reversed in the presence of unique secondary interactions inherent in the molecule; interactions that may 9:45 AM be activated by some property of the reacting partner 5 Common Reducing Agents (Borohydrides) Reduction of Amides to Amines 9:45 AM 6 Common Reducing Agents (Borohydrides) Reduction of Carboxylic Acids to Primary Alcohols O 3 R CO2H + BH3 R O B + 3 H 3 2 Acyloxyborane 9:45 AM 7 Common Reducing Agents (Sodium Borohydride) The reductions with NaBH4 are commonly carried out in EtOH (Serving as a protic solvent) Note that nucleophilic attack occurs from the least hindered face of the 8 carbonyl Common Reducing Agents (Lithium Borohydride) The reductions with LiBH4 are commonly carried out in THF or ether Note that nucleophilic attack occurs from the least hindered face of the 9:45 AM 9 carbonyl. Common Reducing Agents (Borohydrides) The Influence of Metal Cations on Reactivity As a result of the differences in reactivity between sodium borohydride and lithium borohydride, chemoselectivity of reduction can be achieved by a judicious choice of reducing agent. 9:45 AM 10 Common Reducing Agents (Sodium Cyanoborohydride) 9:45 AM 11 Common Reducing Agents (Reductive Amination with Sodium Cyanoborohydride) 9:45 AM 12 Lithium Aluminium Hydride Lithium aluminiumhydride reacts the same way as lithium borohydride. -

UNIT 6 ELEMENTS of GROUP 13 Structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction Uses I 6.3 General Characteristics
UNIT 6 ELEMENTS OF GROUP 13 structure 6.1 Introduction Objectives I 6.2 Oailcrence, Extraction and Uses Occurrec - Extraction uses i 6.3 General Characteristics I - 6.5 Halides of Bpron anel Aluminium Halides of Boron Halides of Aluminium 6.6 Oxides of Boron and Aluminium I Boric Oxide Aluminium Oxide 6.7 Oxoacids of Boron and Borates 6.8 Borazine 6.9 Complexation Behaviour 6.10 Anomalous Behaviour of Boron 6.11 Summary 6.12 Terminal Questions 6.13- Answers ' -- 6.1 INTRODUCTION In the previous two units, you studied the main features of the chemistry of Group 1 and Group 2 elements, i.e. the alkali and the alkaline earth metals. In this unit you - will study the elements of Group 13, namely, boron, aluminium, gallium, indium and, thallium. While studying the alkali and alkaline earth metals, you have seen that all Zhe elements of these two groups are highly reactive metals and the first element of each group shows some differences from the rest. In Group 13, the differences between the first element and the remaining elements become so pronounced that the first member of the group, i.e. boron is a nonmetal wheieas the rest of the elements are distinctly metallic in nature. In a way, this is the first group of the periodic table in which you observe a marked change in the hature of the elements . down the group. describe the chemistry of hydrides, halides and oxides of boron and aluminium, elucidate the structures of hydrides of boron and aluminium, 6.2 OCCURRENC~,EXTRACTION AND USES Elements bf Group 13 are sufficiently reactive. -

Hydrogen Transmutation of Nickel in Glow Discharge
International Journal of Materials Science ISSN 0973-4589 Volume 12, Number 3 (2017), pp. 405-409 © Research India Publications http://www.ripublication.com Hydrogen Transmutation of Nickel in Glow Discharge Vladimir K. Nevolin National Research University of Electronic Technology (MIET), Moscow, Russia. Abstract Background: The possibility of the existence of subatomic hydrogen states was theoretically predicted previously. Objectives: Prove that the transmutation of elements is possible in specially prepared conditions for hydrogen. Methodology: By comparing the mass spectra of deposits on silicon substrates and target electrodes, it is shown that a change in the composition is observed in a magnetron Argon. Results: An increase in the concentration of 62 60 the nickel isotope 28 Ni and a decrease in the isotope concentration 28 Ni are shown. Conclusion: These results confirm the results obtained earlier in the heat generator Rossi, who worked more than a year, found an increase in the 62 isotope 28 Ni due to a decrease in the proportion of other isotopes. Keywords: transmutation, isotopes of nickel, glow discharge, argon, hydrogen INTRODUCION It is considered that the cold transmutation of elements (cold nuclear reactions) has been experimentally demonstrated [1]. On the basis of this phenomenon, energy generators are created in which long-term release of thermal energy in excess of expended energy is observed [2]. From many experimental studies it can be seen that hydrogen, which plays a pivotal role in the reaction zone, may be delivered through a variety of chemical compounds; for example, using lithium aluminium hydride LiAlH4. An analysis of the products of nuclear reactions suggests the possibility of many simultaneous nuclear fusion and decomposition reactions [3]. -

WO 2015/177807 Al 26 November 2015 (26.11.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/177807 Al 26 November 2015 (26.11.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 403/14 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/IN20 15/000066 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 3 February 2015 (03.02.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 1709/MUM/2014 22 May 2014 (22.05.2014) (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: WANBURY LTD. [IN/IN]; BSEL tech park, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, B wing, 10th floor, sector 30A, opp. -

The Response of Work Function of Thin Metal Films to Interaction with Hydrogen
Vol. 114 (2008) ACTA PHYSICA POLONICA A Supplement Proceedings of the Professor Stefan Mr¶ozSymposium The Response of Work Function of Thin Metal Films to Interaction with Hydrogen R. Du¶s¤, E. Nowicka and R. Nowakowski Institute of Physical Chemistry, Polish Academy of Sciences Kasprzaka 44/52, 01-224 Warszawa, Poland The aim of this paper is to summarize the results of experiments carried out at our laboratory on the response of the work function of several thin ¯lms of transition metals and rare earth metals to interaction with molecular hydrogen. The main focus concerns the description of surface phenomena accompanying the reaction of hydride formation as a result of the adsor- bate's incorporation into the bulk of the thin ¯lms. Work function changes ¢© caused by adsorption and reaction concern the surface, hence this ex- perimental method is appropriate for solving the aforementioned problem. A di®erentiation is made between the work function changes ¢© due to cre- ation of speci¯c adsorption states characteristic of hydrides, and ¢© arising as a result of surface defects and protrusions induced in the course of the reaction. The topography of thin metal ¯lms and thin hydride ¯lms with defects and protrusions was illustrated by means of atomic force microscopy. For comparison, the paper discusses work function changes caused by H2 in- teraction with thin ¯lms of metals which do not form hydrides (for example platinum), or when this interaction is performed under conditions excluding hydride formation for thermodynamic reasons. Almost complete diminishing of ¢© was observed, in spite of signi¯cant hydrogen uptake on some rare earth metals, caused by formation of the ordered H{Y{H surface phase. -

An X-Ray Study of Palladium Hydrides up to 100 Gpa: Synthesis and Isotopic Effects Bastien Guigue, Grégory Geneste, Brigitte Leridon, Paul Loubeyre
An x-ray study of palladium hydrides up to 100 GPa: Synthesis and isotopic effects Bastien Guigue, Grégory Geneste, Brigitte Leridon, Paul Loubeyre To cite this version: Bastien Guigue, Grégory Geneste, Brigitte Leridon, Paul Loubeyre. An x-ray study of palladium hydrides up to 100 GPa: Synthesis and isotopic effects. Journal of Applied Physics, American Institute of Physics, 2020, 127 (7), pp.075901. 10.1063/1.5138697. hal-03024503 HAL Id: hal-03024503 https://hal.archives-ouvertes.fr/hal-03024503 Submitted on 11 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. An x-ray study of palladium hydrides up to 100 GPa: Synthesis and isotopic eects. Bastien Guigue,1, 2 Grégory Geneste,2 Brigitte Leridon,1 and Paul Loubeyre2, a) 1)LPEM, ESPCI Paris, PSL Research University, CNRS, Sorbonne Université, 75005 Paris, France 2)CEA, DAM, DIF, F-91297 Arpajon, France (Dated: 10 December 2020) The stable forms of palladium hydrides up to 100 GPa were investigated using the direct reaction of palladium with hydrogen (deuterium) in a laser-heated diamond anvil cell. The structure and volume of PdH(D)x were measured using synchrotron x-ray diffraction. -

Lithium Aluminium Hydride.Pdf
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 5.0 Revision Date 03/12/2011 Print Date 09/12/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Aluminium lithium hydride Product Number : 531502 Brand : Aldrich Supplier : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # (For : (314) 776-6555 both supplier and manufacturer) Preparation Information : Sigma-Aldrich Corporation Product Safety - Americas Region 1-800-521-8956 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Water Reactive, Target Organ Effect, Toxic by ingestion, Corrosive Target Organs Kidney, Liver, Central nervous system GHS Classification Substances, which in contact with water, emit flammable gases (Category 1) Acute toxicity, Oral (Category 3) Skin corrosion (Category 1B) Serious eye damage (Category 1) GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H260 In contact with water releases flammable gases which may ignite spontaneously. H301 Toxic if swallowed. H314 Causes severe skin burns and eye damage. Precautionary statement(s) P223 Keep away from any possible contact with water, because of violent reaction and possible flash fire. P231 + P232 Handle under inert gas. Protect from moisture. P280 Wear protective gloves/ protective clothing/ eye protection/ face protection. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/ physician. P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. P422 Store contents under inert gas. -

Hydrogen Storage Overview
HYDROGEN STORAGE (Materials) Arturo Fernandez Madrigal Instituto de Energias Renovables-UNAM [email protected] Questions ¿what is the state of the art on hydrogen as energy storage? ¿Which are the main challenges of involved technologies? Hydrogen Economic Hydrogen and other fuels [Sørensen, 2005]. Propiedad Hidrógeno Metanol Metano Propano Gasolina Unidad Mínima energía de 0.02 ---- 0.29 0.25 0.24 mJ ignición Temperatura de 2045 ---- 1875 ---- 2200 °C flama Temperatura de 585 385 540 510 230-500 °C autoignición Máxima velocidad 3.46 ---- 0.43 0.47 ---- m/s de flama Rango de 4-75 7-36 5-15 2.5-9.3 1.0-7.6 Vol. % flamabildad Rango de 13-65 ---- 6.3-13.5 ---- 1.1-3.3 Vol. % explosividad Coeficiente de 0.61 0.16 0.20 0.10 0.05 10-3 m2/s difusión Energy Content of Comparative Fuels Physical storage of H2 •Compressed •Metal Hydride (“sponge”) •Cryogenically liquified •Carbon nanofibers Chemical storage of hydrogen •Sodium borohydride •Methanol •Ammonia •Alkali metal hydrides New emerging methods •Amminex tablets •Solar Zinc production •DADB (predicted) •Alkali metal hydride slurry Compressed •Volumetrically and Gravimetrically inefficient, but the technology is simple, so by far the most common in small to medium sized applications. •3500, 5000, 10,000 psi variants. Liquid (Cryogenic) •Compressed, chilled, filtered, condensed •Boils at 22K (-251 C). •Slow “waste” evaporation •Gravimetrically and volumetrically efficient •Kept at 1 atm or just slightly over. but very costly to compress 9 Metal Hydrides (sponge) •Sold by “Interpower” in Germany •Filled with “HYDRALLOY” E60/0 (TiFeH2) •Technically a chemical reaction, but acts like a physical storage method •Hydrogen is absorbed like in a sponge. -

Azidocinnamates in Heterocyclic Synthesis
AZIDOCINNAMATES IN HETEROCYCLIC SYNTHESIS A THESIS PRESENTED BY DEIRDRE M.B. HICKEY B.SC. FOR THE DEGREE OF DOCTOR OF PHILOSOPHY j UNIVERSITY OF LONDON Hofmann Laboratory, Department of Chemistry, Imperial College of Science and Technology, London, SW7 2AY. October, 1982. To my parents, Paddy and Thevese 3 with love and gratitude. ACKNOWLEDGEMENTS I thank Professor C.W. Rees for his enthusiastic supervision during the course of this work and my co-supervisor, Dr. C.J. Moody for his constant help and encouragement. I am grateful to Mr. P. Sulsh for technical help, to Mr. D. Neuhaus and Mr. R. Sheppard for Bruker n.m.r. spectra, to Mr. K. Jones for the analytical service, to Mr. J. Bilton, Mrs. Lee, and Mr. N. Davies for the mass spectroscopy service, and to Mr. D. Everitt in the Stores. Miss Moira Shanahan has expertly typed this thesis and I am grateful for her patience and perserverance. I thank Mr. Michael Casey for proof-reading this thesis and for his help and friendship for many years. Dr. Brian Bell I thank for his help and continued support during the production of this thesis. My colleagues on the seventh floor I will warmly remember for their friendship and comradeship. Finally, grateful acknowledgement is made to the Department of Chemistry, Imperial College for a research assistantship. iv ABSTRACT The reactions of various types of nitrenes to give heterocyclic products is reviewed. A series of vinyl azides, mostly ort/zo-substituted azidocinnamates, was prepared and their decomposition reactions studied. The thermal decomposition of ortho-alkyl azidocinnamates gave 2,4- disubstituted indoles, 1,3-disubstituted isoquinolines, 1,3-disubstituted -1,2-dihydroisoquinolines, and enamines, the amounts of each varying with the ortho-group and the conditions used, iodine having a marked effect on the product ratios.