Formularies Pricing Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 8,148,546 B2 Schuster Et Al
US008148546B2 (12) United States Patent (10) Patent No.: US 8,148,546 B2 Schuster et al. (45) Date of Patent: Apr. 3, 2012 (54) TETRAHYDROCARBAZOLE DERIVATIVES (58) Field of Classification Search .................. 548/448: ASLGANDS OF G-PROTEIN COUPLED 51474 11 RECEPTORS See application file for complete search history. (75) Inventors: Tilmann Schuster, Grossostheim (DE); Klaus Paulini, Maintal (DE); Peter (56) References Cited Schmidt, Schoeneck (DE); Silke Baasner, Schoeneck (DE); Emmanuel FOREIGN PATENT DOCUMENTS Polymeropoulos, Frankfurt (DE); WO WO O3051837 * 6, 2003 Eckhard Guenther, Maintal (DE); WO WO 2006005484 * 1, 2006 Michael Teifel, Weiterstadt (DE) OTHER PUBLICATIONS (73) Assignee: AEterna Zentaris GmbH, Frankfurt Kubinyi (3D QSAR in Drug Design: Ligand-Protein Interactions and (DE) Molecular Similarity, vol. 2-3, Springer, 1998, 800 pages), TOC, pp. 243-244 provided.* *) NotOt1Ce: Subjubject to anyy d1Sclaimer,disclai theh term off thisthi Tatsuta et al. (Bioorg. Med. Chem. Lett. 15 (2005) 2265-2269).* patent is extended or adjusted under 35 Wermuth, The Practice of Medicinal Chemsitry, 2d ed. (2003), 768 U.S.C. 154(b) by 852 days. pages, chs. 9-10 provided.* CAPLUS Abstract of WO O3051837.* (21) Appl. No.: 12/109,479 * cited by examiner (22) Filed: Apr. 25, 2008 (65) Prior Publication Data Primary Examiner — Robert Havlin (74) Attorney, Agent, or Firm — Oblon, Spivak, US 2009/O 170783 A1 Jul. 2, 2009 McClelland, Maier & Neustadt, L.L.P. Related U.S. Application Data (60) Provisional application No. 60/914,424, filed on Apr. (57) ABSTRACT 27, 2007. The present invention provides novel tetrahydrocarbazole compounds according to formula (I) as ligands of G-protein (30) Foreign Application Priority Data coupled receptors (GPCR) which are useful in the treatment and/or prophylaxis of physiological and/or pathological con Apr. -

Yerevan State Medical University After M. Heratsi
YEREVAN STATE MEDICAL UNIVERSITY AFTER M. HERATSI DEPARTMENT OF PHARMACY Balasanyan M.G. Zhamharyan A.G. Afrikyan Sh. G. Khachaturyan M.S. Manjikyan A.P. MEDICINAL CHEMISTRY HANDOUT for the 3-rd-year pharmacy students (part 2) YEREVAN 2017 Analgesic Agents Agents that decrease pain are referred to as analgesics or as analgesics. Pain relieving agents are also called antinociceptives. An analgesic may be defined as a drug bringing about insensibility to pain without loss of consciousness. Pain has been classified into the following types: physiological, inflammatory, and neuropathic. Clearly, these all require different approaches to pain management. The three major classes of drugs used to manage pain are opioids, nonsteroidal anti-inflammatory agents, and non opioids with the central analgetic activity. Narcotic analgetics The prototype of opioids is Morphine. Morphine is obtained from opium, which is the partly dried latex from incised unripe capsules of Papaver somniferum. The opium contains a complex mixture of over 20 alkaloids. Two basic types of structures are recognized among the opium alkaloids, the phenanthrene (morphine) type and the benzylisoquinoline (papaverine) type (see structures), of which morphine, codeine, noscapine (narcotine), and papaverine are therapeutically the most important. The principle alkaloid in the mixture, and the one responsible for analgesic activity, is morphine. Morphine is an extremely complex molecule. In view of establish the structure a complicated molecule was to degrade the: compound into simpler molecules that were already known and could be identified. For example, the degradation of morphine with strong base produced methylamine, which established that there was an N-CH3 fragment in the molecule. -

Supplementary Digital Content REVIEW of ANXIETY MODELS USED in 2010-2011. Type of the Study. Here We Cathegorize the Study Accor
Supplementary digital content REVIEW OF ANXIETY MODELS USED IN 2010-2011. LEGEND Type of the study. Here we cathegorize the study according to its major aim or major finding as follows: anxiogenic effects, the compound had effects opposite to expectations; detection of anxiolytic effect, anxiolytic effects were noticed but no suggestion was formulated for use as anxiolytics; established anxiolytic, well-known anxiolytics studied for various reasons; no effect on anxiety, the compound did not fulfil expectations; mechanism, studies employing anxiolytic treatments but addressing developmental issues, neural mechanisms, drug interactions etc.; putative novel anxiolytic, the authors suggested the compound for anxiolytic drug development; (H), the tested compound was an herbal extract; (Ho), purified or synthesized compound of herbal origin. Anxiety tests. The tests listed in Table 1 were considered 'classical' for the reasons specified in the Abstract. Modifier. Tests are sometimes performed under unconventional conditions to induce heightened levels of anxiety and by this to icrease the translational value of the test. Procedures that altered (usually increased) anxiety levels normally shown in the given test were categorized as follows. chemical, hormonal or pharmacological treatments (e.g. ovarectomy, drug administration in adolescence, etc.); condition, modified testing conditions (e.g. high lighting, no habituation to the testing room, etc.); neural, treatments that affect neural functions (e.g. brain lesions, inhibited neurogenesis); selection, subjects from selection lines, specific strains, etc; stress, subjects exposed to stressors with long-lasting consequences (e.g. chronic immobility, drug withdrawal, etc); subject, the use of specific subjects classes (aged subjects, females, etc.); transgenic, the effects of drugs were studied in genetically engineered subjects. -

Download Product Insert (PDF)
PRODUCT INFORMATION Semax Item No. 27719 CAS Registry No.: 80714-61-0 N Formal Name: L-methionyl-L-α-glutamyl- N H S L-histidyl-L-phenylalanyl-L- H2N prolylglycyl-L-proline O Synonym: ACTH4-7-PGP N H N N O MF: C37H51N9O10S O FW: 813.9 H N OH N O H Purity: ≥95% N O O O Supplied as: A solid H Storage: -20°C O HO Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Semax is supplied as a solid. A stock solution may be made by dissolving the semax in the solvent of choice, which should be purged with an inert gas. Semax is soluble in organic solvents such as DMSO and dimethyl formamide. The solubility of semax in these solvents is approximately 5 and 1 mg/ml, respectively. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of semax can be prepared by directly dissolving the solid in aqueous buffers. The solubility of semax in PBS, pH 7.2, is approximately 10 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description Semax is a synthetic peptide analog of adrenocorticotropic hormone (ACTH) (4-10) (Item No. 27106) that has neuroprotective, analgesic, and anxiolytic properties.1-3 In vivo, semax (0.3 mg/kg) reduces cortical nitric oxide (NO) production and the number of neurological disturbances, such as seizures, falling, and twisting, in a rat model of global ischemia.1 It decreases acetic acid-induced writhing and nociception in a hind paw compression test in mice when administered at doses ranging from 0.015 to 0.5 mg/kg.2 Semax also increases time spent in the open arms in the elevated plus maze, indicating anxiolytic activity, in a rat model of maternal deprivation-induced anxiety.3 References 1. -

Effects of Semax on Dopaminergic and Serotoninergic Systems of the Brain K
Doklady Biological Sciences, Vol. 394, 2004, pp. 1–3. Translated from Doklady Akademii Nauk, Vol. 394, No. 1, 2004, pp. 130–132. Original Russian Text Copyright © 2004 by Eremin, Kudrin, Grivennikov, Miasoedov, Rayevsky. PHYSIOLOGY Effects of Semax on Dopaminergic and Serotoninergic Systems of the Brain K. O. Eremin*, V. S. Kudrin*, I. A. Grivennikov**, Academician N. F. Miasoedov**, and K. S. Rayevsky* Received June 11, 2003 Short-term effects of the synthetic nootropic peptide in rats and simultaneously raises the intracellular level Semax on the content and metabolism of monoamines of dopamine in the striatum [6]. in the brain of C57/Bl6 mice and Sprague–Dawley rats The goal of this study was to examine the effects of were studied. Intraperitoneal injection of Semax at a Semax on the neurochemical parameters of the dopam- dose of 0.15 mg/kg caused an increase in the tissue con- inergic and serotoninergic systems of the animal brain. centrations of 5-hydroxyindoleacetic acid (5-HIAA) in Semax was administered at a dose of 0.15 mg per kilo- the hypothalamus and striatum of mice 0.5 and 2 h after gram body weight to ë57/Bl6 mice, and their hypothal- the injection. Using brain microdialysis, we detected amus and striatum were analyzed for tissue levels of increased levels of extracellular 5-HIAA in the striatum dopamine (DA) and its metabolites 3,4-dihydroxyphe- of freely moving rats 1 h after administration of 0.15 or nylacetic acid (DOPAC) and homovanillic acid (HVA), 0.6 mg/kg Semax. The effect lasted for an additional as well as serotonin (or 5-hydroxytriptamine, 5-HT) 3 h. -

(12) United States Patent (10) Patent No.: US 9,345,661 B2 Adler Et Al
USOO9345661 B2 (12) United States Patent (10) Patent No.: US 9,345,661 B2 Adler et al. (45) Date of Patent: May 24, 2016 (54) SUBCUTANEOUSANTI-HER2 ANTIBODY FOREIGN PATENT DOCUMENTS FORMULATIONS AND USES THEREOF CL 2756-2005 10/2005 CL 561-2011 3, 2011 (75) Inventors: Michael Adler, Riehen (CH); Ulla CL 269-2012 1, 2012 Grauschopf, Riehen (CH): CN 101163717 4/2008 Hanns-Christian Mahler, Basel (CH): CN 101370525 2, 2009 Oliver Boris Stauch, Freiburg (DE) EP O590058 B1 11, 2003 EP 1516628 B1 3, 2005 (73) Assignee: Genentech, Inc., South San Francisco, EP 1603541 11, 2009 JP 2007-533631 11, 2007 CA (US) JP 2008-5075.20 3, 2008 JP 2008-528638 T 2008 (*) Notice: Subject to any disclaimer, the term of this JP 2009-504142 2, 2009 patent is extended or adjusted under 35 JP 2009/055343 4/2009 U.S.C. 154(b) by 0 days. KR 2007-0068385 6, 2007 WO 93.21319 A1 10, 1993 WO 94/OO136 A1 1, 1994 (21) Appl. No.: 12/804,703 WO 97.048O1 2, 1997 WO 98.22136 5, 1998 (22) Filed: Jul. 27, 2010 WO 99.57134 11, 1999 WO 01.00245 A2 1, 2001 (65) Prior Publication Data WO 2004/078140 9, 2004 WO 2005/023328 3, 2005 US 2011 FOO44977 A1 Feb. 24, 2011 WO 2005/037992 A2 4/2005 WO 2005,117986 12/2005 (30) Foreign Application Priority Data WO WO 2005,117986 A2 * 12/2005 WO 2006/044908 A2 4/2006 Jul. 31, 2009 (EP) ..................................... 0.9167025 WO 2006/09 1871 8, 2006 WO 2007 O24715 3, 2007 (51) Int. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0337042 A1 Reilly Et Al
US 2015 0337042A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0337042 A1 Reilly et al. (43) Pub. Date: Nov. 26, 2015 (54) ANTI-EGFRANTIBODIES AND ANTIBODY (22) Filed: Mar. 20, 2015 DRUG CONUGATES Related U.S. Application Data (71) Applicant: ABBVIE INC., North Chicago, IL (US) (60) Provisional application No. 61/968,819, filed on Mar. 21, 2014. (72) Inventors: Edward B. Reilly, Libertyville, IL (US); Publication Classification Andrew C. Phillips, Libertyville, IL (US); Lorenzo Benatuil, Northborough, (51) Int. Cl. MA (US); Fritz G. Buchanan, Antioch, C07K 6/28 (2006.01) IL (US); Jonathan A. Meulbroek, Lake A647/48 (2006.01) Bluff, IL (US); Chung-Ming Hsieh, (52) U.S. Cl. Newton, MA (US); Jennifer Perez, CPC ....... C07K 16/2863 (2013.01); A61K 47/48561 (2013.01); C07K 2317/21 (2013.01); C07K Granville, NY (US) 231 7/565 (2013.01) (57) ABSTRACT (73) Assignee: ABBVIE INC., North Chicago, IL (US) The invention relates to anti-epidermal growth factor (EGFR) antibodies and antibody drug conjugates (ADCs), including compositions and methods of using said antibodies and (21) Appl. No.: 14/664,453 ADCs. Patent Application Publication Nov. 26, 2015 Sheet 1 of 24 US 2015/0337042 A1 8HODZHOD|HCJD |?un6|+ Patent Application Publication Nov. 26, 2015 Sheet 4 of 24 US 2015/0337042 A1 FACS Binding to Tumor Cells 1 OOOO 7500 5OOO 25OO O.O1 1 100 1 OOOO Patent Application Publication Nov. 26, 2015 Sheet 5 of 24 US 2015/0337042 A1 009/ ueeu Oed Patent Application Publication Nov. 26, 2015 Sheet 6 of 24 US 2015/0337042 A1 EGFR (1-525) Binding kinetics (closed circles) = Ab1 and Ab2 106 (open circles) = variants Figure 6 Patent Application Publication Nov. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding Et Al
US 20140051633A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding et al. (43) Pub. Date: Feb. 20, 2014 (54) HEPATOCYTE GROWTH FACTOR MIMICS (60) Provisional application No. 61/706,567, filed on Sep. AS THERAPEUTICAGENTS 27, 2012, provisional application No. 61/706,437, filed on Sep. 27, 2012. (71) Applicant: Washington State University, Pullman, WA (US) Publication Classification (72) Inventors: Joseph W. Harding, Pullman, WA (US); John W. Wright, Pullman, WA (US); (51) Int. C. Caroline C. Benoist, Nashville, TN C07K5/065 (2006.01) (US); Leen H. Kawas, Pullman, WA (52) U.S. C. (US); Gary A. Wayman, Pullman, WA CPC .................................. C07K5/06078 (2013.01) (US) USPC ........................................................... S14/95 (73) Assignee: Washington State University, Pullman, WA (US) (57) ABSTRACT (21) Appl. No.: 14/038,973 (22) Filed: Sep. 27, 2013 Small molecule, peptidic hepatocyte growth factors mimics, which act as both mimetics and antagonists, have been gen Related U.S. Application Data erated. These molecules have been shown or predicted to have (63) Continuation-in-part of application No. PCT/US2012/ therapeutic potential for numerous pathologies including 031815, filed on Apr. 2, 2012. dementia (e.g. Alzheimer's) and Parkinson's disease. Patent Application Publication Feb. 20, 2014 Sheet 1 of 40 US 2014/0051633 A1 a --- --- - - -s ------- CS -8- Scopolarine s -k- Oii exa-ow " -: N. Sks s i. Y s re. Dihexa-high as: ^ N.-- st-scs:- -------------------------------------------------------------------------------------------. Acquisition (days) Figure 1A -8-CSF -- -8- Scopoanine i. --- - -k- Dihexa-ow -- hexa-high reta-or 2 3 4 5 6 7 8 Acquisition (days) Figure 1B -8- vici-treated -- hexa Acquisition (days) Figure 1C Patent Application Publication Feb. -
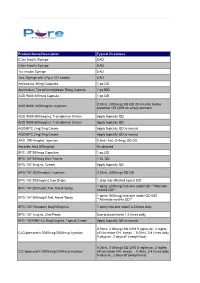
Pure Pricing List
Product Name/Description Typical Directions 0.3cc Insulin Syringe UAD 0.5cc Insulin Syringe UAD 1cc Insulin Syringe UAD 1mL Syringe with 27g x 1/2" needle UAD Amlexanox 40mg Capsules 1 po QD Ammonium Tetrathiomolybdate 50mg Capsule 1 po BID AOD 9604 600mcg Capsule 1 po QD 0.25mL (300mcg) SQ QD 30 minutes before AOD 9604 1200mcg/mL Injection breakfast OR QHS on empty stomach AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD/BPC 2mg/2mg Cream Apply topically QD to wound AOD/BPC 2mg/2mg Cream Apply topically QD to wound ARA 290 4mg/mL Injection 0.5mL-1mL (2-4mg) SQ QD Ascorbic Acid 500mg/mL As directed BPC-157 500mcg Capsules 1 po QD BPC-157 500mcg Mini-Troche 1 SL QD BPC-157 2mg/mL Cream Apply topically QD BPC-157 2000mcg/mL Injection 0.25mL (500mcg) SQ QD BPC-157 200mcg/mL Eye Drops 1 drop into affected eye(s) BID 1 spray (200mcg) into one nostril QD **Alternate BPC-157 200mcg/0.1mL Nasal Spray nostrils QD** 1 spray (500mcg) into one nostril QD-BID BPC-157 500mcg/0.1mL Nasal Spray **Alternate nostrils QD** BPC-157/ Synapsin 5mg/50mg/mL 1 spray into one nostril 2-3 times daily BPC-157 1mg/mL Oral Paste Use to brush teeth 1-2 times daily BPC-157/GHK-Cu 2mg/2mg/mL Topical Cream Apply topically QD to wound 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, sleep)… 0.05mL 3-4 times daily 5 days on, 2 days off (weight loss) 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, -

Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats
G C A T T A C G G C A T genes Article Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats Ivan B. Filippenkov 1,* , Vasily V. Stavchansky 1, Alina E. Denisova 2, Vadim V. Yuzhakov 3, Larisa E. Sevan’kaeva 3, Olga Y. Sudarkina 1 , Veronika G. Dmitrieva 1, Leonid V. Gubsky 2, Nikolai F. Myasoedov 1, Svetlana A. Limborska 1 and Lyudmila V. Dergunova 1 1 Institute of Molecular Genetics, Russian Academy of Sciences, 123182 Moscow, Russia; [email protected] (V.V.S.); [email protected] (O.Y.S.); [email protected] (V.G.D.); [email protected] (N.F.M.); [email protected] (S.A.L.); [email protected] (L.V.D.) 2 Pirogov Russian National Research Medical University, Federal Center of Cerebrovascular Pathology and Stroke, Ministry of Health Care of Russian Federation, 117342 Moscow, Russia; [email protected] (A.E.D.); [email protected] (L.V.G.) 3 A. Tsyb Medical Radiological Research Center–Branch of the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, 249031 Obninsk, Russia; [email protected] (V.V.Y.); [email protected] (L.E.S.) * Correspondence: fi[email protected]; Tel.: +7-499-196-1858 Received: 16 May 2020; Accepted: 18 June 2020; Published: 22 June 2020 Abstract: Cerebral ischaemia is the most common cause of impaired brain function. Biologically active peptides represent potential drugs for reducing the damage that occurs after ischaemia. The synthetic melanocortin derivative, ACTH(4-7)PGP (Semax), has been used successfully in the treatment of patients with severe impairment of cerebral blood circulation. -

Counteracting EMF Toxicity with Peptides (Forget Global Warming Emfs Are Killing Us)
Counteracting EMF Toxicity with Peptides (Forget Global Warming EMFs are Killing Us) Dr. Kent Holtorf, MD Medical Director/CEO, Holtorf Medical Group (HoltorfMed.com) Medical Director/CEO, National Academy of Hypothyroidism (NAHypothyroidism.org) Medical Director, Integrative Peptides (IntegrativePeptides.com) Disclosure Statement Owner/CEO - Holtorf Medical Group HoltorfMed.com A multi-specialty medical group specializing in the treatment of complex endocrine dysfunction, CFS/fibromyalgia, chronic infectious diseases, immune dysfunction, neurodegenerative disease, and other complex chronic illnesses. Chief Medical Officer - The Non-Profit, The National Academy of Hypothyroidism and Integrative Sciences (NAHIS) NAHypothyroidism.org NAHIS is a non- profit, multidisciplinary medical society dedicated to the dissemination of new information on the diagnosis and treatment of hypothyroidism and complex conditions. NAHIS receives grants for clinical and laboratory research for novel methods in the diagnosis and treatment of hypothyroidism and chronic illnesses Chief Medical Officer - Integrative Peptides IntegrativePeptides.com Currently sells orally active and absorbable natural peptides in oral supplement form with unique delivery methods 2 Disclosure Statement Important Disclaimer In the interest of full disclosure, I am the All studies have limitations, and there is no perfect study. As with other studies, the studies Chief Medical Officer of Integrative Peptides. mentioned in this presentation and during the summit have limitations that should be considered when evaluating the efficacy of any treatment, including peptides, and determining the The opinions expressed are mine and do not appropriateness of peptide therapy for consumer use. A short summary, the abstract or reference to necessarily reflect those of Integrative a study may be discussed or provided. We are happy to provide you the entire study for your review of any study discussed, mentioned, presented or referenced. -

PEPTIDE INFORMATION Contact Kitt Burkley for Pricing 855-762-4878
PEPTIDE INFORMATION Contact Kitt Burkley for pricing 855-762-4878 AOD 9604 AOD 9604 works by mimicking the way natural HGH regulates fat metabolism but without the adverse effects on blood sugar or growth that is seen with unmodified HGH. Studies have suggested that AOD 9604 has an ability to stimulate lipolysis and inhibit lipogenesis. In addition AOD 9604 possesses other regenerative properties associated with growth hormone. Trials have been undertaken to determine the possible application of AOD 9604 in osteoarthritis and hypercholesterolemia, as well as for bone and cartilage repair. Results of oral glucose tolerance testing have demonstrated that AOD 9604 has no negative effect on carbohydrate metabolism, and it does not affect serum IGF-1 levels. AOD 9604 has an excellent safety profile and is generally well tolerated. It has attained Human GRAS status in the US. AOD 9604 600mcg/ml Cream – 30g AOD 9604* 0.5mg Troche BPC-157 (Body Protection Compound) BPC-157 promotes muscle and tendon healing, increases angiogenesis and decreases inflammatory response. Produces more type 1 collagen and has excellent potential for diabetic wound healing. BPC- 157 is highly stable, resistant to both hydrolysis and enzyme digestion. It can be given orally as well as administered subcutaneously or intramuscularly – no carrier molecule is required. This peptide has significant anti-inflammatory modulating effects. BPC-157 promotes tissue healing through signaling pathways – it provides specific response to particular injury thru cell signaling. BPC- 157 can be used continuously as body protective, or for specific period to treat body injury. BCP-157 is in clinical trials for IBD.