Product Catalogwells Pharmacy Network
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Joy Wellness Partners 838 W G Street Suite 201 San Diego, California 92101
United States of America FEDERAL TRADE COMMISSION Southwest Region 1999 Bryan St., Ste. 2150 Dallas, Texas 75201 June 3, 2020 WARNING LETTER VIA EMAIL TO [email protected] Joy Wellness Partners 838 W G Street Suite 201 San Diego, California 92101 Re: Unsubstantiated Claims for Coronavirus Prevention or Treatment To Whom It May Concern, This is to advise you that FTC staff reviewed your website at https://joywellnesspartners.com/ on May 24, 2020. We have determined that you are unlawfully advertising that certain products treat or prevent Coronavirus Disease 2019 (COVID-19). Some examples of Coronavirus treatment or prevention claims on your website include: In marketing materials accessible on your website by selecting “BLOG” from the navigation menu and clicking on “CORONAVIRUS (COVID-19),” you claim: o Under the heading “Hormones, Peptides, & Immunity”: . You state that “Keeping your immune system running optimally with balanced hormones and peptides helps your body prevent and fight illness, including viruses like COVID-19. Book now [https://intakeq.com/booking/8hkwjk]” . You post a video titled “IMMUNITY, BIOTE, & CORONAVIRUS” featuring Carol Joy Bender, NP. In the video at approximately 31:55, Bender states, “More specifically, I wanted to touch on the things that we’re all concerned about the coronavirus times: what can I do to boost my immune system to keep me healthy? BioTE has already been doing this for us… We have a lot of patients that are already taking their iodine with selenium and zinc. The zinc is one of the mainstay treatments that many of you have read about… zinc helps to reduce the RNA replication inside the cells, when the coronavirus gets in. -

Endogenous Peptide Discovery of the Rat Circadian Clock a FOCUSED STUDY of the SUPRACHIASMATIC NUCLEUS by ULTRAHIGH PERFORMANCE TANDEM MASS □ SPECTROMETRY* S
Research Endogenous Peptide Discovery of the Rat Circadian Clock A FOCUSED STUDY OF THE SUPRACHIASMATIC NUCLEUS BY ULTRAHIGH PERFORMANCE TANDEM MASS □ SPECTROMETRY* S Ji Eun Lee‡§, Norman Atkins, Jr.¶, Nathan G. Hatcherʈ, Leonid Zamdborg‡§, Martha U. Gillette§¶**, Jonathan V. Sweedler‡§¶ʈ, and Neil L. Kelleher‡§‡‡ Understanding how a small brain region, the suprachias- pyroglutamylation, or acetylation. These aspects of peptide matic nucleus (SCN), can synchronize the body’s circa- synthesis impact the properties of neuropeptides, further ex- dian rhythms is an ongoing research area. This important panding their diverse physiological implications. Therefore, time-keeping system requires a complex suite of peptide unveiling new peptides and unreported peptide properties is hormones and transmitters that remain incompletely critical to advancing our understanding of nervous system characterized. Here, capillary liquid chromatography and function. FTMS have been coupled with tailored software for the Historically, the analysis of neuropeptides was performed analysis of endogenous peptides present in the SCN of the rat brain. After ex vivo processing of brain slices, by Edman degradation in which the N-terminal amino acid is peptide extraction, identification, and characterization sequentially removed. However, analysis by this method is from tandem FTMS data with <5-ppm mass accuracy slow and does not allow for sequencing of the peptides con- produced a hyperconfident list of 102 endogenous pep- taining N-terminal PTMs (5). Immunological techniques, such tides, including 33 previously unidentified peptides, and as radioimmunoassay and immunohistochemistry, are used 12 peptides that were post-translationally modified with for measuring relative peptide levels and spatial localization, amidation, phosphorylation, pyroglutamylation, or acety- but these methods only detect peptide sequences with known lation. -

Thymosin Hh10 Inhibits Angiogenesis and Tumor Growth by Interfering with Ras Function
Research Article Thymosin hh10 Inhibits Angiogenesis and Tumor Growth by Interfering with Ras Function Seung-Hoon Lee,1 Myung Jin Son,1,2 Sun-Hee Oh,1 Seung-Bae Rho,1 Kyungsook Park,1 Yung-Jin Kim,2 Mi-Sun Park,1 and Je-Ho Lee1 1Molecular Therapy Research Center, Samsung Medical Center, School of Medicine, Sung Kyun Kwan University, Seoul, Korea and 2Department of Molecular Biology, Pusan National University, Busan, Korea Abstract are a family of highly conserved small peptides that inhibit barbed end actin polymerization by sequestering actin mono- Thymosin h10 is a monomeric actin sequestering protein mers (8). Among them, thymosin h4 and thymosin h10 are the that regulates actin dynamics. Previously, we and others h have shown that thymosin h acts as an actin-mediated two most abundant -thymosins in the mammalian species and 10 coexist in some tissue types at varying ratios (9). Although both tumor suppressor. In this study, we show that thymosin h10 is not only a cytoskeletal regulator, but that it also acts as a peptides share a high degree of sequence homology, they show potent inhibitor of angiogenesis and tumor growth by its distinct patterns of expression in several tissues (10) and play interaction with Ras. We found that overexpressed thymosin different roles during rodent development (11). Recently, the angiogenic effects of several members of the thymosin family of h10 significantly inhibited vascular endothelial growth factor–induced endothelial cell proliferation, migration, peptides were studied in the chick chorioallantoic membrane h a invasion, and tube formation in vitro. Vessel sprouting was model (12). -

Preclinical Effects of Melanocortins in Male Sexual Dysfunction
International Journal of Impotence Research (2008) 20, S11–S16 & 2008 Nature Publishing Group All rights reserved 0955-9930/08 $30.00 www.nature.com/ijir Preclinical effects of melanocortins in male sexual dysfunction AM Shadiack1 and S Althof2 1Locus Pharmaceuticals, Blue Bell, PA, USA and 2The Center for Marital and Sexual Health of South Florida, West Palm Beach, FL, USA The neurobiology of sexual behavior involves the interrelationships between sex steroids and neurotransmitters that result in both central nervous system (CNS) effects and effects in the genitalia. Tools such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) scanning can help determine what areas of the brain are activated under sexual stimulation. Our understanding of the role of various neurotransmitters, neurosteroids and other CNS-acting compounds is improving. The role of CNS-acting compounds such as dopamine agonists in the treatment of male sexual dysfunction is under active investigation. Melanocortins have CNS and peripheral roles in a wide variety of bodily functions. The melanocortin agonist bremelanotide appears to act in the CNS to promote erections in preclinical models, and may also stimulate behaviors that facilitate sexual activity beyond their erectogenic effects. International Journal of Impotence Research (2008) 20, S11–S16; doi:10.1038/ijir.2008.17 Keywords: erectile dysfunction; neuroanatomy; neurophysiology; CNS-acting agents; melanocortins; bremelanotide Introduction Neuroanatomy of male sexual response The neurobiology of sexual behavior involves the Sex and the brain interrelationships between sex steroids and neuro- Sexual arousal can now be studied with such transmitters that result in both central nervous sophisticated tools as PET (positron emission system (CNS) effects and effects in the genitalia. -

Multiple Beneficial Effects of Melanocortin MC4 Receptor
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca - Università degli studi di Napoli Federico II Progress in Neurobiology 148 (2017) 40–56 Contents lists available at ScienceDirect Progress in Neurobiology journal homepage: www.elsevier.com/locate/pneurobio Review article Multiple beneficial effects of melanocortin MC4 receptor agonists in experimental neurodegenerative disorders: Therapeutic perspectives a a a b c Daniela Giuliani , Alessandra Ottani , Laura Neri , Davide Zaffe , Paolo Grieco , d e f a, Jerzy Jochem , Gian Maria Cavallini , Anna Catania , Salvatore Guarini * a Department of Biomedical, Metabolic and Neural Sciences, Section of Pharmacology and Molecular Medicine, University of Modena and Reggio Emilia, Modena, Italy b Department of Biomedical, Metabolic and Neural Sciences, Section of Human Morphology, University of Modena and Reggio Emilia, Modena, Italy c Department of Pharmacy, University of Napoli “Federico II”, Napoli, Italy d Department of Basic Medical Sciences, School of Public Health in Bytom, Medical University of Silesia, Katowice, Poland e Department of Ophthalmology, University of Modena and Reggio Emilia, Modena, Italy f Center for Preclinical Surgical Research, Fondazione IRCCS Ca' Granda À Ospedale Maggiore Policlinico, Milano, Italy A R T I C L E I N F O A B S T R A C T Article history: Received 20 May 2015 Melanocortin peptides induce neuroprotection in acute and chronic experimental neurodegenerative Received in revised form 22 November 2016 conditions. Melanocortins likewise counteract systemic responses to brain injuries. Furthermore, they Accepted 28 November 2016 promote neurogenesis by activating critical signaling pathways. Melanocortin-induced long-lasting Available online 1 December 2016 improvement in synaptic activity and neurological performance, including learning and memory, sensory-motor orientation and coordinated limb use, has been consistently observed in experimental Keywords: models of acute and chronic neurodegeneration. -

Supporting Information for Proteomics DOI 10.1002/Pmic.200700142
Supporting Information for Proteomics DOI 10.1002/pmic.200700142 Karl Skld, Marcus Svensson, Mathias Norrman, Benita Sjgren, Per Svenningsson and Per E. Andren´ The significance of biochemical and molecular sample integrity in brain proteomics and peptidomics: Stathmin 2-20 and peptides as sample quality indicators ª 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.proteomics-journal.com SUPPORTING INFORMATION Supporting Information Table 1. Degraded protein identities and peptide sequences in the striatum after 1, 3, and 10 min post-mortem. UniProtKBa. Protein name Sequenceb Scorec P60710/P63260 Actin, cytoplasmic 1,2 A.LVVDNGSGMCK.A 56 E.MATAASSSSLEKS.Y 55 W.IGGSILASLSTFQQ.M 64 W.ISKQEYDESGPSIVHRK.C 93 M.WISKQEYDESGPSIVHRK.C 56 Q8K021 Secretory carrier-associated F.ATGVMSNKTVQTAAANAASTAATSAAQNAFKGNQM.- 124 membrane protein 1 Q9D164 FXYD domain-containing ion L.ITTNAAEPQK.A 58 transport regulator 6 precursor L.ITTNAAEPQKA.E 57 L.ITTNAAEPQKAE.N 89 L.ITTNAAEPQKAEN.- 54 P99029 Peroxiredoxin 5, mitochondrial M.APIKVGDAIPSVEVF.E 57 precursor P01942 Hemoglobin alpha subunit F.LASVSTVLTSKYR.- 106 M.FASFPTTKTYFPHF.D 72 L.ASHHPADFTPAVHASLDK.F 76 T.LASHHPADFTPAVHASLDK.F 59 L.LVTLASHHPADFTPAVHAS.L 56 L.LVTLASHHPADFTPAVHASLDK.F 71 L.LVTLASHHPADFTPAVHASLDKFLASVST.V 66 T.LASHHPADFTPAVHASLDKFLAS.V 55 L.VTLASHHPADFTPAVHASLDKFLAS.V 68 -.VLSGEDKSNIKAAWGKIGGHGAEYGAEALER.M 97 -.VLSGEDKSNIKAAWGKIGGHGAEYGAEALERM.F 58 P02088/P02089 Hemoglobin beta-1,2 subunit L.LVVYPWTQRY.F 53 L.LVVYPWTQRYF.D 52 Q00623 Apolipoprotein A-I precursor Y.VDAVKDSGRDYVSQFESSSLGQQLN.L -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. A Bell & Howell Information Company 300 North Zeeb Road. Ann Arbor. Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 9517090 Partial characterization and purification of steroidogenic factors in thymic epithelial cell culture-conditioned medium Uzumcu, Mehmet, Ph.D. The Ohio State University, 1994 UMI 300 N. -

Stabilization Stabilizor Workflow Inactivation of Enzymes
Denator Novel Tissue Sample Preparation Technology for MS Analysis denator.com WATERS 2nd NORDIC MS SYMPOSIUM Tuesday Nov 8 2011 Båstad Katarina Alenäs Denator 2004 University spin-off 2006 Head office at Biotech Center Gothenburg, Sweden Research lab in Uppsala Science Park, Sweden 2008 Stabilizor T1 – Launch 2009-to date Publications: Svensson et al. “Heat stabilization of the tissue proteome” Journal of Proteome Research, 2009 Robinson et al. “Assessing the use of thermal treatment to preserve the intact proteomes of post-mortem heart and brain tissue” Proteomics, 2009 Scholz et al. “Impact of temperature dependent sampling procedures in proteomics and peptidomics” Molecular and Cellular Proteomics, 2010 Goodwin et al. “Stopping the clock on proteomic degradation by heat-treatment at the point of tissue excision” Proteomics, 2010 Rountree et al “Clinical application for the preservation of phospho-proteins through in-situ tissue stabilization” Proteome Science, 2010 Ahmed et al “Preserving protein profiles in tissue samples: Differing outcomes with and without heat stabilization” Journal of Neuroscience Methods, 2011 Colgrave et al “Neuropeptide profiling of the bovine hypothalamus: Thermal stabilization is an effective tool in inhibiting post-mortem degradation” Colgrave et al, Proteomics, 2011 Protein research workflow Collection Sample prep Analysis Bioinformatics Stabilization Extraction 2D-gels Dissection Clean up MS Storage Enrichment Western blot Transport Trypsin cleavage ELISA Laboratory for Biological and Medical Mass Spectrometry, -

(12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding Et Al
US 20140051633A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding et al. (43) Pub. Date: Feb. 20, 2014 (54) HEPATOCYTE GROWTH FACTOR MIMICS (60) Provisional application No. 61/706,567, filed on Sep. AS THERAPEUTICAGENTS 27, 2012, provisional application No. 61/706,437, filed on Sep. 27, 2012. (71) Applicant: Washington State University, Pullman, WA (US) Publication Classification (72) Inventors: Joseph W. Harding, Pullman, WA (US); John W. Wright, Pullman, WA (US); (51) Int. C. Caroline C. Benoist, Nashville, TN C07K5/065 (2006.01) (US); Leen H. Kawas, Pullman, WA (52) U.S. C. (US); Gary A. Wayman, Pullman, WA CPC .................................. C07K5/06078 (2013.01) (US) USPC ........................................................... S14/95 (73) Assignee: Washington State University, Pullman, WA (US) (57) ABSTRACT (21) Appl. No.: 14/038,973 (22) Filed: Sep. 27, 2013 Small molecule, peptidic hepatocyte growth factors mimics, which act as both mimetics and antagonists, have been gen Related U.S. Application Data erated. These molecules have been shown or predicted to have (63) Continuation-in-part of application No. PCT/US2012/ therapeutic potential for numerous pathologies including 031815, filed on Apr. 2, 2012. dementia (e.g. Alzheimer's) and Parkinson's disease. Patent Application Publication Feb. 20, 2014 Sheet 1 of 40 US 2014/0051633 A1 a --- --- - - -s ------- CS -8- Scopolarine s -k- Oii exa-ow " -: N. Sks s i. Y s re. Dihexa-high as: ^ N.-- st-scs:- -------------------------------------------------------------------------------------------. Acquisition (days) Figure 1A -8-CSF -- -8- Scopoanine i. --- - -k- Dihexa-ow -- hexa-high reta-or 2 3 4 5 6 7 8 Acquisition (days) Figure 1B -8- vici-treated -- hexa Acquisition (days) Figure 1C Patent Application Publication Feb. -
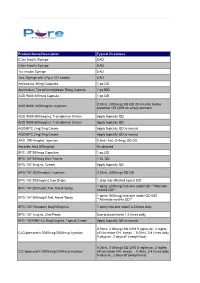
Pure Pricing List
Product Name/Description Typical Directions 0.3cc Insulin Syringe UAD 0.5cc Insulin Syringe UAD 1cc Insulin Syringe UAD 1mL Syringe with 27g x 1/2" needle UAD Amlexanox 40mg Capsules 1 po QD Ammonium Tetrathiomolybdate 50mg Capsule 1 po BID AOD 9604 600mcg Capsule 1 po QD 0.25mL (300mcg) SQ QD 30 minutes before AOD 9604 1200mcg/mL Injection breakfast OR QHS on empty stomach AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD/BPC 2mg/2mg Cream Apply topically QD to wound AOD/BPC 2mg/2mg Cream Apply topically QD to wound ARA 290 4mg/mL Injection 0.5mL-1mL (2-4mg) SQ QD Ascorbic Acid 500mg/mL As directed BPC-157 500mcg Capsules 1 po QD BPC-157 500mcg Mini-Troche 1 SL QD BPC-157 2mg/mL Cream Apply topically QD BPC-157 2000mcg/mL Injection 0.25mL (500mcg) SQ QD BPC-157 200mcg/mL Eye Drops 1 drop into affected eye(s) BID 1 spray (200mcg) into one nostril QD **Alternate BPC-157 200mcg/0.1mL Nasal Spray nostrils QD** 1 spray (500mcg) into one nostril QD-BID BPC-157 500mcg/0.1mL Nasal Spray **Alternate nostrils QD** BPC-157/ Synapsin 5mg/50mg/mL 1 spray into one nostril 2-3 times daily BPC-157 1mg/mL Oral Paste Use to brush teeth 1-2 times daily BPC-157/GHK-Cu 2mg/2mg/mL Topical Cream Apply topically QD to wound 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, sleep)… 0.05mL 3-4 times daily 5 days on, 2 days off (weight loss) 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, -

Counteracting EMF Toxicity with Peptides (Forget Global Warming Emfs Are Killing Us)
Counteracting EMF Toxicity with Peptides (Forget Global Warming EMFs are Killing Us) Dr. Kent Holtorf, MD Medical Director/CEO, Holtorf Medical Group (HoltorfMed.com) Medical Director/CEO, National Academy of Hypothyroidism (NAHypothyroidism.org) Medical Director, Integrative Peptides (IntegrativePeptides.com) Disclosure Statement Owner/CEO - Holtorf Medical Group HoltorfMed.com A multi-specialty medical group specializing in the treatment of complex endocrine dysfunction, CFS/fibromyalgia, chronic infectious diseases, immune dysfunction, neurodegenerative disease, and other complex chronic illnesses. Chief Medical Officer - The Non-Profit, The National Academy of Hypothyroidism and Integrative Sciences (NAHIS) NAHypothyroidism.org NAHIS is a non- profit, multidisciplinary medical society dedicated to the dissemination of new information on the diagnosis and treatment of hypothyroidism and complex conditions. NAHIS receives grants for clinical and laboratory research for novel methods in the diagnosis and treatment of hypothyroidism and chronic illnesses Chief Medical Officer - Integrative Peptides IntegrativePeptides.com Currently sells orally active and absorbable natural peptides in oral supplement form with unique delivery methods 2 Disclosure Statement Important Disclaimer In the interest of full disclosure, I am the All studies have limitations, and there is no perfect study. As with other studies, the studies Chief Medical Officer of Integrative Peptides. mentioned in this presentation and during the summit have limitations that should be considered when evaluating the efficacy of any treatment, including peptides, and determining the The opinions expressed are mine and do not appropriateness of peptide therapy for consumer use. A short summary, the abstract or reference to necessarily reflect those of Integrative a study may be discussed or provided. We are happy to provide you the entire study for your review of any study discussed, mentioned, presented or referenced. -

PEPTIDE INFORMATION Contact Kitt Burkley for Pricing 855-762-4878
PEPTIDE INFORMATION Contact Kitt Burkley for pricing 855-762-4878 AOD 9604 AOD 9604 works by mimicking the way natural HGH regulates fat metabolism but without the adverse effects on blood sugar or growth that is seen with unmodified HGH. Studies have suggested that AOD 9604 has an ability to stimulate lipolysis and inhibit lipogenesis. In addition AOD 9604 possesses other regenerative properties associated with growth hormone. Trials have been undertaken to determine the possible application of AOD 9604 in osteoarthritis and hypercholesterolemia, as well as for bone and cartilage repair. Results of oral glucose tolerance testing have demonstrated that AOD 9604 has no negative effect on carbohydrate metabolism, and it does not affect serum IGF-1 levels. AOD 9604 has an excellent safety profile and is generally well tolerated. It has attained Human GRAS status in the US. AOD 9604 600mcg/ml Cream – 30g AOD 9604* 0.5mg Troche BPC-157 (Body Protection Compound) BPC-157 promotes muscle and tendon healing, increases angiogenesis and decreases inflammatory response. Produces more type 1 collagen and has excellent potential for diabetic wound healing. BPC- 157 is highly stable, resistant to both hydrolysis and enzyme digestion. It can be given orally as well as administered subcutaneously or intramuscularly – no carrier molecule is required. This peptide has significant anti-inflammatory modulating effects. BPC-157 promotes tissue healing through signaling pathways – it provides specific response to particular injury thru cell signaling. BPC- 157 can be used continuously as body protective, or for specific period to treat body injury. BCP-157 is in clinical trials for IBD.