Preclinical Effects of Melanocortins in Male Sexual Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uses of Bremelanotide in Therapy for Female Sexual
(19) TZZ __T (11) EP 2 916 856 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) A61K 38/12 (2006.01) 19.09.2018 Bulletin 2018/38 A61P 15/00 (2006.01) C07K 7/64 (2006.01) (21) Application number: 13851014.4 (86) International application number: PCT/US2013/068386 (22) Date of filing: 05.11.2013 (87) International publication number: WO 2014/071339 (08.05.2014 Gazette 2014/19) (54) USES OF BREMELANOTIDE IN THERAPY FOR FEMALE SEXUAL DYSFUNCTION VERWENDUNG VON BREMELANOTID IN DER THERAPIE VON WEIBLICHER SEXUELLER DYSFUNKTION UTILISATIONS DE BRÉMÉLANOTIDE DANS UNE THÉRAPIE DE DYSFONCTIONNEMENT SEXUEL FÉMININ (84) Designated Contracting States: • "Palatin Technologies Presents Positive Results AL AT BE BG CH CY CZ DE DK EE ES FI FR GB for Phase 2B Bremelanotide Female Sexual GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Dysfunction Trial", PRNewswire , 8 November PL PT RO RS SE SI SK SM TR 2012 (2012-11-08), XP002757483, Retrieved from the Internet: (30) Priority: 05.11.2012 US 201261722511 P URL:http://www.palatin.com/investors/press 28.02.2013 US 201361770535 P -releases/ [retrieved on 2016-05-09] • ROSEN R C ET AL: "Evaluation of the safety, (43) Date of publication of application: pharmacokineticsand pharmacodynamic effects 16.09.2015 Bulletin 2015/38 of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male (73) Proprietor: Palatin Technologies, Inc. subjects and in patients with an inadequate Cranbury NJ 08512 (US) response to Viagra(R)", INTERNATIONAL JOURNAL OF IMPOTENCE RESEARCH, (72) Inventors: STOCKTON, BASINGSTOKE, GB, vol. -

Modi Dissertation 2012 Submitted To
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: ________________________ ______________________ Meera Modi Date Identifying Novel Therapeutic Strategies for Enhancing Social Cognition Using Functional Animal Models By Meera Modi Doctor of Philosophy Graduate Division of Biological and Biomedical Science Neuroscience _________________________________________ Larry Young, PhD Advisor _________________________________________ Lisa Parr, PhD Committee Member _________________________________________ Diana Robins, PhD Committee Member _________________________________________ Randy Hall, PhD Committee Member _________________________________________ Kerry J. Ressler, MD/PhD Committee Member Accepted: _________________________________________ Lisa A. Tedesco, Ph.D. Dean of the James T. Laney School of Graduate Studies ___________________ Date Identifying Novel Therapeutic -

Female Sexual Interest / Arousal Disorder
Female Sexual Interest / See SSRI sexual dysfunction Arousal Disorder decision support Asse ss for me dica tions w hich Refer to appropriate SSRI may affect sexual function History of physical/sexual/emotional abuse Present psychosocial services other (i.e SSRI, TCA, contraceptives, and/or intimate partner violence? and agencies available Benzodiazepines, antipsychotics) in community Reassess need for medication and transition if Substance use Appropriate Evaluate for partner Obtain full medical/sexual/ disorder? Present See corresponding Sexual dysfunction psychosocial history decision support Comorbid psychiatric Present disorder present? Treat underlyingPresent Evaluate for non-gynecological cause and reassess medical etiology Commorbidity absent Evaluate for gynecological etiology Symptom Is duration greater than 6 months Symptom persistence and causing significant distre ss? persistence Yes Female with Sexual Inte re st Arousa l Disorder Asse ss a nd e sta blish treatment expectations a nd goals Behavior Modification: Lifestyle changes Psychotherapy: Body image Sexual therapy Consider Sleep hygiene Trailored intervention Couples therapy combination therapy Stress reduction Based on H&P Mindfulness Pelvic therapy Relationship building Sexual novelty Inadequate response Continue Psychotherapy and Behavior modification interventions Inadequate response Consider topical oils (i.e. Zestra) in addition to Bupropion Pharmacotherapy Inadequate response Buspirone is less studied, however, it may be useful in some circumstances Postmenopausal? Buspirone No Yes Consider testosterone Flibanserin therapy. Consider consultation Inadequate response Bremelanotide FEMALE SEXUAL INTEREST /AROUSAL DISORDER Bupropion XL Antidepressant DOSING: starting dose 150mg XL qAM. Increase after 1 week to 300mg XL Wellbutrin XL (dopamine and qAM. Target dose 300mg- 450mg XL qAM. Max dose 450mg XL qAM. norepinephrine uptake inhibitor) SIDE EFFECTS: (common) insomnia, nausea, agitation, tremor (rare, serious) seizures, especially in patients with a history of seizures or eating disorders. -

Bremelanotide in HSDD Presented at the 22Nd Annual Fall Scientific Meeting of 1 2 2 2 3 Sexual Medicine Society of North America Anita H
222 The Neurobiology and Efficacy of Bremelanotide in HSDD Presented at the 22nd Annual Fall Scientific Meeting of 1 2 2 2 3 Sexual Medicine Society of North America Anita H. Clayton, Johna Lucas, Robert Jordan, Carl Spana, James G. Pfaus November 3– 6, 2016 Scottsdale, AZ 1University of Virginia, Charlottesville, VA, USA; 2Palatin Technologies, Cranbury, NJ, USA; 3Concordia University, Montréal, Québec, Canada • Introduction Mechanism of Sexual and Reward Circuitry Rat Study Human Study — Decreased (less distress) both the total score and • These neurochemicals act on similar regions of the brain, • BMT’s downstream CNS effects of increasing arousal • Among premenopausal women with HSDD with or scores on the desire, arousal, and orgasm items of • Female sexual dysfunctions are classified as dysfunctions Sexual Response such as the medial preoptic area (mPOA) in the hypothalamus, and desire are thought to result from its action as a without FSAD, subcutaneous (SC) administration of the FSDS–Desire-Arousal-Orgasm measure. Mean of desire (eg, hypoactive sexual desire disorder [HSDD]), attention- and reward-related regions of the limbic system, MC-receptor agonist BMT 1.25 or 1.75 mg: (SE) change, –11.1 (12.0) vs –6.8 (13.6); BMT vs arousal (eg, female sexual arousal disorder [FSAD]), • Excitatory signals are regulated by dopamine (DA), placebo, respectively ( P=0.0014; Figure 7 )11 and the prefrontal cortex • Among female rats primed with estrogen and proges - — Significantly increased the number of sexually satisfying delay or absence of orgasm, or sexual pain (dyspareunia norepinephrine (NE), oxytocin, and the melanocortins 5,6 — Gamma aminobutyric acid projections help regulate DA terone or estrogen alone, BMT significantly increased events (SSEs) vs placebo when taken 45 minutes or vaginismus) 1 (MCs) Figure 7. -

Conversations with Women About Female Sexual Dysfunction (FSD)
235 Conversations With Women About Female Sexual Dysfunction (FSD) and Treatment With Bremelanotide Presented at the 22nd Annual Fall Scientific Meeting of 1 2 3 4 5 5 6 Sexual Medicine Society of North America Patricia E. Koochaki , Stanley Althof , Sheryl A. Kingsberg , Michael A. Perelman , Johna Lucas , Robert Jordan , Dennis A. Revicki November 3– 6, 2016 Scottsdale, AZ 1PEK Market Research, Inc., Cincinnati, OH, USA; 2Case Western Reserve University School of Medicine, Cleveland, OH, USA; 3University Hospitals Case Medical Center, Cleveland, OH, USA; 4Weill Cornell Medical College, New York, NY, USA; 5Palatin Technologies, Cranbury, NJ, USA; Evidera, Bethesda, MD, USA • Recognizing There Was a Problem Partner and Relationship Effects Physical Effects of BMT Sexual Satisfaction Increased Summary of Benefits That Women With HSDD With/Without Introduction Results Decreased Arousal During the interviews, women described how they Partner Blamed Himself • About three-quarters of all participants reported experiencing Approximately two-thirds of participants taking 1.25 and 1.75 mg Female sexual dysfunctions (FSD) include a Pre-Study: Conceptualizing and Explaining Why began to recognize they were experiencing sexual “There were a couple of times where he did blame himself. He physical effects from BMT of BMT reported an improvement in sexual satisfaction. range of distressing, multifactorial conditions, They Experienced FSD difficulties that required intervention. thought something was wrong with him.” • Nine of 10 women taking the 1.75-mg dose of BMT reported “A positive way …I think it restored or rebuilt what [sexual such as dysfunctions of sexual interest/desire, • Most women were unaware that FSD is a recognized “Because for a young man our age, he’s thinking like, it must be me. -

VYLEESI Is Not These Highlights Do Not Include All the Information Needed to Use Recommended in Patients at High Risk for Cardiovascular Disease
HIGHLIGHTS OF PRESCRIBING INFORMATION treatment and ensure blood pressure is well-controlled. VYLEESI is not These highlights do not include all the information needed to use recommended in patients at high risk for cardiovascular disease. (5.1) VYLEESI™ safely and effectively. See full prescribing information for • Focal hyperpigmentation: Reported by 1% of patients who received up to 8 VYLEESI doses per month, including involvement of the face, gingiva and breasts. Higher risk in patients with darker skin and with daily dosing. Resolution VYLEESI (bremelanotide injection), for subcutaneous use was not confirmed in some patients. Consider discontinuing VYLEESI if Initial U.S. Approval: 2019 hyperpigmentation develops. (5.2) • Nausea: Reported by 40% of patients who received up to 8 monthly doses, ----------------------------INDICATIONS AND USAGE-------------------------- requiring anti-emetic therapy in 13% of patients and leading to premature VYLEESI is a melanocortin receptor agonist indicated for the treatment of discontinuation for 8% of patients. Improved for most patients with the premenopausal women with acquired, generalized hypoactive sexual desire second dose. Consider discontinuing VYLEESI or initiating anti-emetic disorder (HSDD) as characterized by low sexual desire that causes marked therapy for persistent or severe nausea. (5.3) distress or interpersonal difficulty and is NOT due to: • A co-existing medical or psychiatric condition, -------------------------------ADVERSE REACTIONS---------------------------- • Problems with the relationship, or Most common adverse reactions (incidence > 4%) are nausea, flushing, • The effects of a medication or drug substance (1). injection site reactions, headache, and vomiting. (6.1) Limitations of Use (1): To report SUSPECTED ADVERSE REACTIONS, contact AMAG • Not indicated for treatment of HSDD in postmenopausal women or in men. -
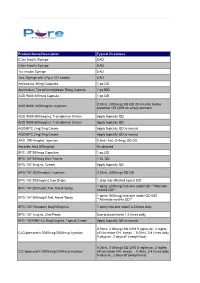
Pure Pricing List
Product Name/Description Typical Directions 0.3cc Insulin Syringe UAD 0.5cc Insulin Syringe UAD 1cc Insulin Syringe UAD 1mL Syringe with 27g x 1/2" needle UAD Amlexanox 40mg Capsules 1 po QD Ammonium Tetrathiomolybdate 50mg Capsule 1 po BID AOD 9604 600mcg Capsule 1 po QD 0.25mL (300mcg) SQ QD 30 minutes before AOD 9604 1200mcg/mL Injection breakfast OR QHS on empty stomach AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD/BPC 2mg/2mg Cream Apply topically QD to wound AOD/BPC 2mg/2mg Cream Apply topically QD to wound ARA 290 4mg/mL Injection 0.5mL-1mL (2-4mg) SQ QD Ascorbic Acid 500mg/mL As directed BPC-157 500mcg Capsules 1 po QD BPC-157 500mcg Mini-Troche 1 SL QD BPC-157 2mg/mL Cream Apply topically QD BPC-157 2000mcg/mL Injection 0.25mL (500mcg) SQ QD BPC-157 200mcg/mL Eye Drops 1 drop into affected eye(s) BID 1 spray (200mcg) into one nostril QD **Alternate BPC-157 200mcg/0.1mL Nasal Spray nostrils QD** 1 spray (500mcg) into one nostril QD-BID BPC-157 500mcg/0.1mL Nasal Spray **Alternate nostrils QD** BPC-157/ Synapsin 5mg/50mg/mL 1 spray into one nostril 2-3 times daily BPC-157 1mg/mL Oral Paste Use to brush teeth 1-2 times daily BPC-157/GHK-Cu 2mg/2mg/mL Topical Cream Apply topically QD to wound 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, sleep)… 0.05mL 3-4 times daily 5 days on, 2 days off (weight loss) 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, -

Estimulos Para Aumentar Estimulos Para Aumentar La Respuesta Sexual
ESTIMULOS PARA AUMENTAR LA RESPUESTA SEXUAL FEMENINA Dr. Santiago Palacios Instituto Palacios, Salud y Medicina de la Mujer Chairman of CAMS (Council of Affiliated Menopause Societies) President of SIBOMM (Ibero American Society of Osteology and Mineral Metabolism) Antonio Acuña, 9 - 28009 Madrid Teléfono 91 578 05 17 E-mail: [email protected] www.institutopalacios.com www.institutopalacios.com Diapositivas / Slides CONTENIDO • ESTUPEFACTO • QUÉ ES LA RESPUESTA SEXUAL FEMENINA • CONOCER LA RESPUESTA SEXUAL • QUÉ TENEMOS • QUÉ SE ESTÁ HACIENDO PARA EL FUTURO * DOCTOR, ACUDO A USTED YA QUE ES ESPECIALISTA EN ESTE CAMPO, PORQUE DESEO AUMENTAR MI RESPUESTA SEXUAL CONSULTA MÉDICA: ¿QUÉ? ¿QUÉ LE DIGO? ¿QUÉ HAGO? CONTENIDO • ESTUPEFACTO • QUÉ ES LA RESPUESTA SEXUAL FEMENINA • CONOCER LA RESPUESTA SEXUAL • QUÉ TENEMOS • QUÉ SE ESTÁ HACIENDO PARA EL FUTURO * Ciclo de respuesta sexual femenina: Modelo lineal • En 1966, Masters y Johnson introdujeron el primer modelo de la función sexual femenina. Propusieron un modelo lineal de sexualidad para mujeres consistente en cuatro etapas: excitación, meseta, orgasmo y resolución. • Diferentes mujeres diferentes patrones de respuesta • Misma mujer diferente patrón de respuesta en diferentes ocasiones Orgasmo Meseta Excitación Masters WH, Johnson V. (1966) Human Sexual Response. Boston: Little, Brown & Co Ciclo de respuesta sexual femenina: Modelo no lineal • En 2001, Basson construyó un modelo no lineal de la respuesta sexual femenina. • El modelo alternativo de Basson incorpora una motivación basada en la intimidad, los estímulos sexuales, los factores confluyentes biológicos y psicológicos y la satisfacción. Intimidad Buscar y ser emocional receptivo Satisfacción emocional y física Impulso Estímul sexual os espontáneo sexuale s Excitación y deseo sexual Deseo Biológic sexual o PiPsico lóilógico Basson R. -

Let's Start a Conversation
LET’S START A CONVERSATION Low Sexual Desire in Women LET’S START A CONVERSATION This educational activity is jointly providedVaginal by the North and Carolina Sexual Academy of Family Health Physicians at(NCAFP) Midlife and Spire Learning. This activity is supported by an educational funding donation provided by AMAG Pharmaceuticals, Inc. LET’S START A CONVERSATION Low Sexual Desire in Women PROGRAM OVERVIEW A lack of interest in sex, or decreased desire, is a common female sexual problem that often goes unrecognized. Hypoactive sexual desire disorder (HSDD) is characterized by diminished feelings of sexual interest or desire that in turn causes distress. HSDD is estimated to affect from 10% to 46% of women in the United States, including women of all ages, regardless of menopausal status. This live meeting series will provide the latest updates in the diagnosis and management of HSDD to help improve the quality of life for your female patients, and will provide insights into: • Recognizing the prevalence ofLET’S HSDD andSTART barriers A toCONVERSATION care • Diagnosis using updated criteria, sexualVaginal health and Sexual communication Health at Midlife strategies, and validated screeners • The importance of identifying and treating common causes and comorbidities of low sexual desire • The range of nonpharmacologic, approved, and emerging pharmacologic and device-based therapies for HSDD TARGET AUDIENCE Family physicians LEARNING OBJECTIVES At the conclusion of this live activity, family physicians should be better able to: • Define -

WO 2017/151816 Al 8 September 2017 (08.09.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/151816 Al 8 September 2017 (08.09.2017) P O P C T (51) International Patent Classification: Gilead Apollo, LLC, 333 Lakeside Drive, Foster City, C07D 495/04 (2006.01) A61P 3/00 (2006.01) California 94404 (US). YANG, Xiaowei; c/o Gilead A61K 31/519 (2006.01) A61P 3/06 (2006.01) Apollo, LLC, 333 Lakeside Drive, Foster City, California 94404 (US). (21) International Application Number: PCT/US20 17/020271 (74) Agents: TANNER, Lorna L. et al; Sheppard Mullin Richter & Hampton LLP, 379 Lytton Avenue, Palo Alto, (22) Date: International Filing California 94301-1479 (US). 1 March 2017 (01 .03.2017) (81) Designated States (unless otherwise indicated, for every (25) Filing Language: English kind of national protection available): AE, AG, AL, AM, (26) Publication Language: English AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, (30) Priority Data: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 62/302,755 2 March 2016 (02.03.2016) US HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, 62/303,237 3 March 2016 (03.03.2016) US KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, (71) Applicant: GILEAD APOLLO, LLC [US/US]; 333 MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, Lakeside Drive, Foster City, California 94404 (US). -

Pharmacological Chaperone Action in Humanized Mouse Models of MC4R-Linked Obesity
Pharmacological chaperone action in humanized mouse models of MC4R-linked obesity Patricia René, … , Denis Richard, Michel Bouvier JCI Insight. 2021;6(4):e132778. https://doi.org/10.1172/jci.insight.132778. Technical Advance Metabolism Therapeutics MC4R mutations represent the largest monogenic cause of obesity, resulting mainly from receptor misfolding and intracellular retention by the cellular quality control system. The present study aimed at determining whether pharmacological chaperones (PCs) that restore folding and plasma membrane trafficking by stabilizing near native protein conformation may represent valid therapeutic avenues for the treatment of melanocortin type 4 receptor–linked (MC4R- linked) obesity. To test the therapeutic PC potential, we engineered humanized MC4R (hMC4R) mouse models expressing either the WT human MC4R or a prevalent obesity-causing mutant (R165W). Administration of a PC able to rescue cell surface expression and functional activity of R165W-hMC4R in cells restored the anorexigenic response of the R165W-hMC4R obese mice to melanocortin agonist, providing a proof of principle for the therapeutic potential of MC4R- targeting PCs in vivo. Interestingly, the expression of the WT-hMC4R in mice revealed lower sensitivity of the human receptor to α–melanocyte-stimulating hormone (α-MSH) but not β-MSH or melanotan II, resulting in a lower penetrance obese phenotype in the WT-hMC4R versus R165W-hMC4R mice. In conclusion, we created 2 new obesity models, a hypomorphic highlighting species differences and an amorphic providing a preclinical model to test the therapeutic potential of PCs to treat MC4R-linked obesity. Find the latest version: https://jci.me/132778/pdf TECHNICAL ADVANCE Pharmacological chaperone action in humanized mouse models of MC4R-linked obesity Patricia René,1 Damien Lanfray,2 Denis Richard,2 and Michel Bouvier1 1Départment de Biochimie et de Médecine Moléculaire, Institute for Research in Immunology and Cancer, Université de Montréal, Montréal, Quebec, Canada. -

Bremelanotide (Vyleesi) Reference Number: ERX.SPA.347 Effective Date: 12.01.19 Last Review Date: 11.20 Line of Business: Commercial, Medicaid Revision Log
Clinical Policy: Bremelanotide (Vyleesi) Reference Number: ERX.SPA.347 Effective Date: 12.01.19 Last Review Date: 11.20 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Bremelanotide (Vyleesi™) is a melanocortin receptor agonist. FDA Approved Indication(s) Vyleesi is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD) as characterized by low sexual desire that causes marked distress or interpersonal difficulty and NOT due to: • A co-existing medical or psychiatric condition, • Problems with the relationship, or • The effects of a medication or drug substance. Limitation(s) of use: • Not indicated for treatment of HSDD in postmenopausal women or in men. • Not indicated to enhance sexual performance. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. Health plan approved formularies should be reviewed for all coverage determinations. Requirements to use preferred alternative agents apply only when such requirements align with the health plan approved formulary. It is the policy of health plans affiliated with Envolve Pharmacy Solutions™ that Vyleesi is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Hypoactive Sexual Desire Disorder (must meet all): 1. Diagnosis of HSDD in premenopausal women; 2. Age ≥ 18 years; 3. Failure of a 3-month trial of bupropion at up to maximally studied effective doses (see Appendix B), unless contraindicated or clinically significant adverse effects are experienced; 4. Vyleesi is not prescribed concurrently with Addyi; 5.