(Oncology & Haematology) Made in Its 110Th Meeting Held On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pertuzumab and Trastuzumab: the Rationale Way to Synergy
Anais da Academia Brasileira de Ciências (2016) 88(1 Suppl.): 565-577 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201620150178 www.scielo.br/aabc Pertuzumab and trastuzumab: the rationale way to synergy SANDRINE RICHARD1, FRÉDÉRIC SELLE1, JEAN-PIERRE LOTZ1,2, AHMED KHALIL1, JOSEPH GLIGOROV1,2 and DANIELE G. SOARES1 1Medical Oncology Department, APREC (Alliance Pour la Recherche En Cancérologie), Tenon Hospital (Hôpitaux Universitaires de l’Est-Parisien, AP-HP), rue de la Chine, 75020 Paris, France 2Institut Universitaire de Cancérologie Université Pierre et Marie Curie (IUC-UPMC Univ Paris 06), Sorbonne Universités, 4 place Jussieu, 75005 Paris, France Manuscript received on March 13, 2015; accepted for publication on May 5, 2015 ABSTRACT It has now been 15 years since the HER2-targeted monoclonal antibody trastuzumab was introduced in clinical and revolutionized the treatment of HER2-positive breast cancer patients. Despite this achievement, most patients with HER2-positive metastatic breast cancer still show progression of their disease, highlighting the need for new therapies. The continuous interest in novel targeted agents led to the development of pertuzumab, the first in a new class of agents, the HER dimerization inhibitors. Pertuzumab is a novel recombinant humanized antibody directed against extracellular domain II of HER2 protein that is required for the heterodimerization of HER2 with other HER receptors, leading to the activation of downstream signalling pathways. Pertuzumab combined with trastuzumab plus docetaxel was approved for the first-line treatment of patients with HER2-positive metastatic breast cancer and is currently used as a standard of care in this indication. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
cancers Review Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies Karam Khaddour 1,*, Sushma Jonna 1, Alexander Deneka 2 , Jyoti D. Patel 3, Mohamed E. Abazeed 4, Erica Golemis 2 , Hossein Borghaei 5 and Yanis Boumber 3,6,* 1 Division of Hematology and Oncology, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] 2 Fox Chase Cancer Center, Program in Molecular Therapeutics, Philadelphia, PA 19111, USA; [email protected] (A.D.); [email protected] (E.G.) 3 Robert H. Lurie Comprehensive Cancer Center, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 4 Robert H. Lurie Comprehensive Cancer Center, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 5 Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA 19111, USA; [email protected] 6 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia * Correspondence: [email protected] (K.K.); [email protected] (Y.B.) Simple Summary: Epidermal growth factor receptor (EGFR) mutations occur in a significant number Citation: Khaddour, K.; Jonna, S.; of lung cancer patients. Treatment outcomes in this subset of patients has greatly improved over the Deneka, A.; Patel, J.D.; Abazeed, M.E.; last decade after the introduction of EGFR tyrosine kinase inhibitors (TKIs), which demonstrated high Golemis, E.; Borghaei, H.; Boumber, Y. efficacy and improved survival in randomized clinical trials. Although EGFR TKIs became the stan- Targeting the Epidermal Growth dard of care in patients with EGFR-mutated lung cancer, resistance almost inevitably develops. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -

Dual Targeting of HER2-Positive Cancer with Trastuzumab Emtansine and Pertuzumab: Critical Role for Neuregulin Blockade in Antitumor Response to Combination Therapy
Published OnlineFirst October 4, 2013; DOI: 10.1158/1078-0432.CCR-13-0358 Clinical Cancer Cancer Therapy: Clinical Research See related article by Gwin and Spector, p. 278 Dual Targeting of HER2-Positive Cancer with Trastuzumab Emtansine and Pertuzumab: Critical Role for Neuregulin Blockade in Antitumor Response to Combination Therapy Gail D. Lewis Phillips1, Carter T. Fields1, Guangmin Li1, Donald Dowbenko1, Gabriele Schaefer1, Kathy Miller5, Fabrice Andre6, Howard A. Burris III8, Kathy S. Albain9, Nadia Harbeck10, Veronique Dieras7, Diana Crivellari11, Liang Fang2, Ellie Guardino3, Steven R. Olsen3, Lisa M. Crocker4, and Mark X. Sliwkowski1 Abstract Purpose: Targeting HER2 with multiple HER2-directed therapies represents a promising area of treatment for HER2-positive cancers. We investigated combining the HER2-directed antibody–drug con- jugate trastuzumab emtansine (T-DM1) with the HER2 dimerization inhibitor pertuzumab (Perjeta). Experimental Design: Drug combination studies with T-DM1 and pertuzumab were performed on cultured tumor cells and in mouse xenograft models of HER2-amplified cancer. In patients with HER2- positive locally advanced or metastatic breast cancer (mBC), T-DM1 was dose-escalated with a fixed standard pertuzumab dose in a 3þ3 phase Ib/II study design. Results: Treatment of HER2-overexpressing tumor cells in vitro with T-DM1 plus pertuzumab resulted in synergistic inhibition of cell proliferation and induction of apoptotic cell death. The presence of the HER3 ligand, heregulin (NRG-1b), reduced the cytotoxic activity of T-DM1 in a subset of breast cancer lines; this effect was reversed by the addition of pertuzumab. Results from mouse xenograft models showed enhanced antitumor efficacy with T-DM1 and pertuzumab resulting from the unique antitumor activities of each agent. -

Colony Stimulating Factors
Market Applicability Market GA KY MD NJ NY Applicable X NA X X X Colony Stimulating Factors Overrides Approval Duration Prior Authorization 1 year Quantity Limit Medications Quantity Limit Fulphila (pegfligrastim-jmdb) May be subject to quantity limit Granix (tbo-filgrastim) N/A Leukine (sargramsotim) N/A Neulasta (pegfilgrastim) May be subject to quantity limit Neupogen (filgrastim) N/A Nivestym (filgrastim-aafi) N/A Nyvepria (pegfilgrastim-apgf) May be subject to quantity limit Udenyca (pegfilgrastim-cbqv) May be subject to quantity limit Zarxio (filgrastim-sndz) N/A Ziextenzo (pegfilgrastim-bmez) May be subject to quantity limit APPROVAL CRITERIA I. *In addition to criteria outlined below, requests for Granix, Leukine, Neupogen, Nivestym, must also meet the following criteria: A. Individual has had a trial and inadequate response or intolerance to Zarxio; OR B. Zarxio is not FDA-approved for the prescribed indication and Granix, Leukine, Neupogen or Nivestym is. *Step Therapy does not apply to Florida Healthy Kids II. Requests for filgrastim (Neupogen), filgrastim-aafi (Nivestym), or filgrastim-sndz (Zarxio) may be approved if the following criteria are met: A. Individual with nonmyeloid malignancy is using for primary prophylaxis of Febrile Neutropenia (FN); AND B. Individual has a risk of FN of 20% or greater based on chemotherapy regimen (see Appendix, Table 1); OR C. Individual with nonmyeloid malignancy is using for primary prophylaxis of FN; AND D. Individual’s risk of developing FN is greater than or equal to 10% and less than 20% based on chemotherapy regimen (see Appendix, Table 1) and individual has any risk factors for: PAGE 1 of 18 02/01/2021 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -
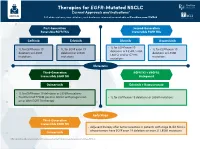
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

Changing the Destiny of HER2 Expressing Solid Tumors
International Journal of Molecular Sciences Review Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors Alice Indini 1 , Erika Rijavec 1 and Francesco Grossi 2,* 1 Medical Oncology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (A.I.); [email protected] (E.R.) 2 Medical Oncology Unit, Department of Medicine and Surgery, University of Insubria, ASST dei Sette Laghi, 21100 Varese, Italy * Correspondence: [email protected] or [email protected] Abstract: HER2 targeted therapies have significantly improved prognosis of HER2-positive breast and gastric cancer. HER2 overexpression and mutation is the pathogenic driver in non-small cell lung cancer (NSCLC) and colorectal cancer, however, to date, there are no approved HER2-targeted therapies with these indications. Trastuzumab deruxtecan (T-DXd) is a novel HER2-directed antibody drug conjugate showing significant anti-tumor activity in heavily pre-treated HER2-positive breast and gastric cancer patients. Preliminary data have shown promising objective response rates in patients with HER2-positive NSCLC and colorectal cancer. T-DXd has an acceptable safety profile, however with concerns regarding potentially serious treatment-emergent adverse events. In this review we focus on the pharmacologic characteristics and toxicity profile of T-Dxd, and provide an update on the most recent results of clinical trials of T-DXd in solid tumors. The referenced papers were selected through a PubMed search performed on 16 March 2021 with the following searching terms: T-DXd and breast cancer, or gastric cancer, or non-small cell lung cancer (NSCLC), or colorectal cancer. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency.