Intramuscular Neural Distribution of the Sartorius Muscles: Treating Spasticity with Botulinum Neurotoxin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elbow Checklist
Workbook Musculoskeletal Ultrasound September 26, 2013 Shoulder Checklist Long biceps tendon Patient position: Facing the examiner Shoulder in slight medial rotation; elbow in flexion and supination Plane/ region: Transverse (axial): from a) intraarticular portion to b) myotendinous junction (at level of the pectoralis major tendon). What you will see: Long head of the biceps tendon Supraspinatus tendon Transverse humeral ligament Subscapularis tendon Lesser tuberosity Greater tuberosity Short head of the biceps Long head of the biceps (musculotendinous junction) Humeral shaft Pectoralis major tendon Plane/ region: Logitudinal (sagittal): What you will see: Long head of biceps; fibrillar structure Lesser tuberosity Long head of the biceps tendon Notes: Subscapularis muscle and tendon Patient position: Facing the examiner Shoulder in lateral rotation; elbow in flexion/ supination Plane/ region: longitudinal (axial): full vertical width of tendon. What you will see: Subscapularis muscle, tendon, and insertion Supraspinatus tendon Coracoid process Deltoid Greater tuberosity Lesser tuberosity Notes: Do passive medial/ lateral rotation while examining Plane/ region: Transverse (sagittal): What you will see: Lesser tuberosity Fascicles of subscapularis tendon Supraspinatus tendon Patient position: Lateral to examiner Shoulder in extension and medial rotation Hand on ipsilateral buttock Plane/ region: Longitudinal (oblique sagittal) Identify the intra-articular portion of biceps LH in the transverse plane; then -

Pes Anserinus Syndrome
DEPARTMENT OF ORTHOPEDIC SURGERY SPORTS MEDICINE Marc R. Safran, MD Professor, Orthopaedic Surgery Chief, Division of Sports Medicine PES ANSERINUS SYNDROME DESCRIPTION The pes anserinus is the tendon insertion of 3 muscles of the thigh into the upper leg (tibia), just below the knee to the inner side of the front of the leg. Where the tendon attaches to bone, there is a bursa sac between the bone and the tendon. The bursa functions like a water balloon to reduce friction and wear of the tendon against the bone. With this syndrome there is inflammation and pain of the bursa (bursitis), tendon (tendinitis), or both. FREQUENT SIGNS AND SYMPTOMS Pain, tenderness, swelling, warmth and/or redness over the pes anserinus bursa and tendon on the front inner leg just 2-3 inches below the knee. The pain is usually slight when beginning to exercise and is worse as the activity continues. Pain with running or bending the knee against resistance Crepitation (a crackling sound) when the tendon or bursa is moved or touched CAUSES Strain from sudden increase in amount or intensity of activity or overuse of the lower extremity usually in the endurance athlete or the athlete just beginning to run. May also be due to direct trauma to the upper leg. RISK INCREASES WITH Endurance sports (distance runs, triathlons) Beginning a training program Sports that require pivoting, cutting (sudden change of direction while running), jumping and deceleration. Incorrect training techniques that include excessive hill running, recent large increases in mileage, inadequate time for rest between workouts.. Poor physical conditioning (strength/flexibility) Inadequate warm-up prior to practice or play Knock knees Arthritis of the knee. -

Keycard M11 21 22.Qxd
M11, M21, M22 ® M11 4 M11 12 5 13 6 14 7 15 8 16 9 17 10 18 11 19 M21 20 M21 28 21 29 22 30 23 31 24 32 25 33 26 34 27 35 M22 36 M22 44 37 45 38 46 39 47 40 48 41 49 42 50 43 51 Latin 11 1 Conexus intertendinei 48 Mm. lumbricales manus 2 Mm. interossei dorsales manus 49 M. flexor digitorum superficialis, tendo 10 3 N. radialis 50 Lig. metacarpale transversum superficiale 2 9 8 1 3 7 12 4 A. radialis 51 Aa. digitales palmares communes 4 5 6 5 Retinaculum extensorum 52 Manus, vagina tendinum digitorum 13 6 M. abductor pollicis brevis 53 M. flexor digitorum profundus, tendo } 19 7 M. extensor carpi radialis brevis 54 Vaginae fibrosae, pars anularis 20 14 21 18 8 M. extensor carpi radialis longus 55 N. ulnaris, nn. digitales palmares proprii 22 9 M. brachioradialis 56 Vaginae fibrosae, pars cruciforme 23 17 32 31 30 24 15 10 M. brachialis 57 Arcus palmaris superficialis 33 29 28 26 25 16 27 11 M. deltoideus 58 M. abductor digiti minimi manus 12 M. supraspinatus 59 M. opponens digiti minimi manus 35 36 20 13 Scapula, spina 60 N. ulnaris, ramus superficialis 21 3 18 14 M. trapezius 61 Aa. metatarsales dorsales 34 10 15 15 M. infraspinatus 62 Os metacarpale, caput 8 9 12 32 30 28 24 16 M. latissimus dorsi 63 Aa. digitales dorsales manus 29 13 17 M. teres major 64 Nn. digitales dorsales (manus) } 18 M. -

Vascular Injury Following Harvesting of Hamstring Tendon Graft for Anterior Cruciate Ligament Reconstruction
Central Annals of Orthopedics & Rheumatology Bringing Excellence in Open Access Case Report *Corresponding author Bruno Pavei, Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil, Email: Vascular Injury Following Submitted: 27 February 2018 Accepted: 10 March 2018 Harvesting of Hamstring Published: 12 March 2018 Copyright Tendon Graft for Anterior © 2018 Pavei OPEN ACCESS Cruciate Ligament Keywords • Knee injuries • Anterior cruciate ligament Reconstruction • Vascular injury Bruno Pavei* Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil Abstract Objectives: To describe a rare case of significant vascular injury that occurred following the harvest of hamstring tendon graft for anterior cruciate ligament reconstruction, with the need of repair. Case report: A 30-year-old male soccer player presented with significant swelling and acute pain two hours after anterior cruciate ligament (ACL) reconstruction. Clinical examination and MRI scan showed bleeding, and a large hematoma in the middle of the left thigh. Hemorrhagic contention was only possible after a secondary repair, where a vessel of the sartorius muscle was bleeding profusely 12 hours after the primary surgery. Discussion: The presence of a large number of vessels on the medial side of the knee associated with blind flexor tendons graft hamstring is cause of vascular injury during removal of the hamstring graft. Conclusion: Although uncommon, graft hamstring of flexor tendons can have devastating consequences if some hemostasis procedures are not correctly applied. INTRODUCTION CASE REPORT Anterior cruciate ligament (ACL) is the most commonly A 30-year-old male soccer player presented with an ACL injured ligament of the knee. -

Burt Klos MD Phd Stephan Konijnenberg MD Ultrasound Imaging and Conservative Treatment Follow up Presenter Disclosure Information
Burt Klos MD PhD Stephan Konijnenberg MD Ultrasound imaging and conservative treatment follow up Presenter Disclosure Information Burt Klos disclosed no conflict of interest. Musculoskeletal Ultrasound • US Cuff /bursa • Knee Bakers Cyst • Knee Tendinitis Ultrasound positions prone , supine , hyperflexion Tendon imaging MRI vs MSU Static Dynamic Overview Focus Recognition Learning curve Less detail Interactive Relative value of MRI sports injury • KSSTA 2017 MRI is not reliable in diagnosing of concomitant anterolateral ligament and anterior cruciate ligament injuries of the knee • BM. Devitt et al AUS • KSSTA 2017 High prevalence of Segond Avulsion in MS ultrasound not found with MRI • Klos , Konijnenberg NL Courtesy C vd Hart * * Sport tendon injuries • Achilles tendon • Patella tendon • Pes anserinus Pes anserinus tendino/bursitis IA pathology (hydrops) Osteofyt impingement Endotorsion /Hyperpronation / Overload Researchgate.net femur tibia Ultrasound injection • Image-guided versus blind corticosteroid • injections in adults with shoulder pain: A systematic review • Edmund Soh 2011 BMC • statistically significant greater improvement in shoulder pain and function at 6 weeks after injection with MS Ultrasound • Sinus tarsi US guided injections Mayo Clinic 2010 • MSU 90 % accurate • Blinded injections 35 % accurate • J of Clinical ultrasound 2018 tibia • Pes anserinus bursa injection : • Blind versus US guided injection • 4/ 22 accurate placement in blind . Pes anserinus bursa injection Patella tendinopathy Patella tendinopathy • Tendon -

Anserine Bursitis an Under-Diagnosed, Easily Treatable Cause of Knee Pain He Anserine Bursa Was Initially Called the No-Name-No-Fame by Suzan M
perspectives on pain ADDRESSING PATIENT NEEDS Anserine bursitis An under-diagnosed, easily treatable cause of knee pain he anserine bursa was initially called the no-name-no-fame BY SUZAN M. ATTAR, MD tbursa. The term anserine bur- sitis presently in use was coined by Moshcowitz in 1937, when he first pelvic area, which results in angu- lateral ligament injury or from pes described the condition.1 lation at the knee joint, putting anserine tendonitis. Certain provo- The anserine bursa is located more pressure on the pes anserine cative manoeuvres, however, may medially, 6 cm below the joint line attachment. Secondary causes in- help: between the attachment of the med- clude medial compartment osteo- • tenderness that extends from ial collateral ligament and the con- arthritis of the knee, obesity, direct the anserine bursal area to the joined tendon (see Figures 1 and 2). trauma, abnormal gait, tight ham- joint line is more likely due to Pes anserinus means “goose’s foot” strings and, less commonly, over- inflammation or injury of the and is the anatomic location of the use injury, as in athletics.4 medial collateral ligament conjoined tendons formed by gracilis, • the supine valgus stress test, sartorius and semitendinosus mus- Clinical symptoms which is used to determine the cles in the knee.2 This article will pro- Pain is localized to a well-defined integrity of the medial collateral vide an overview, including the clinical area on the medial knee region over ligament, should not aggravate presentation and management. the upper tibia. Patients often point the pain of anserine bursitis to the spot with one finger. -

Pelvis. Muscles of the Lower Limb. Walking
Pelvis. Muscles of the lower limb. Walking Sándor Katz M.D.,Ph.D. Pelvis Pelvic ligaments Pelvis Hip muscles: iliopsoas (psoas major and iliacus) iliacus Origin: psoas major: vertebral bodies of the T12-L4 vertebrae and costal processes of the L1-5 vertebrae iliacus: iliac fossa Insertion: lesser trochanter Action: flexion of the hip joint; lateral flexion of the lumbar spine Innervation:spinal nerves and femoral nerve Hip muscles - gluteus maximus • Origin: dorsal to the posterior gluteal line of the ilium, sacrum and thoracolumbar fascia • Insertion: gluteal tuberosity, iliotibial tract • Action: Hip joint: adbuction-adduction, extension and lateral rotation. Knee joint: stabilisation when the knee is extended. • Innervation: inferior gluteal nerve Hip muscles - gluteus medius • Origin: between the anterior and posterior gluteal lines of the ilium • Insertion: greater trochanter • Action: abduction and rotation. • Innervation: superior gluteal nerve Hip muscles - gluteus minimus • Origin: between the anterior and inferior gluteal lines of the ilium • Insertion: greater trochanter • Action: abduction and rotation. • Innervation: superior gluteal nerve Hip muscles Piriformis • Origin: 2nd-4th sacral pelvic foramina • Insertion: tip of the greater trochanter • Action: abduction and lateral rotation. • Innervation: sciatic nerve Obturator internus • Origin: inner surface o f t h e o b t u r a t o r foramen and obturator membrane • I n s e r t i o n : trochanteric fossa • Action: adduction and lateral rotation. • Innervation: sacral plexus Obturator externus • Origin: outer surface o f t h e o b t u r a t o r foramen and obturator membrane • I n s e r t i o n : trochanteric fossa • Action: adduction and lateral rotation. -

The Nerves of the Adductor Canal and the Innervation of the Knee: An
REGIONAL ANESTHESIA AND ACUTE PAIN Regional Anesthesia & Pain Medicine: first published as 10.1097/AAP.0000000000000389 on 1 May 2016. Downloaded from ORIGINAL ARTICLE The Nerves of the Adductor Canal and the Innervation of the Knee An Anatomic Study David Burckett-St. Laurant, MBBS, FRCA,* Philip Peng, MBBS, FRCPC,†‡ Laura Girón Arango, MD,§ Ahtsham U. Niazi, MBBS, FCARCSI, FRCPC,†‡ Vincent W.S. Chan, MD, FRCPC, FRCA,†‡ Anne Agur, BScOT, MSc, PhD,|| and Anahi Perlas, MD, FRCPC†‡ pain in the first 48 hours after surgery.3 Femoral nerve block, how- Background and Objectives: Adductor canal block contributes to an- ever, may accentuate the quadriceps muscle weakness commonly algesia after total knee arthroplasty. However, controversy exists regarding seen in the postoperative period, as evidenced by its effects on the the target nerves and the ideal site of local anesthetic administration. The Timed-Up-and-Go Test and the 30-Second Chair Stand Test.4,5 aim of this cadaveric study was to identify the trajectory of all nerves that In recent years, an increased interest in expedited care path- course in the adductor canal from their origin to their termination and de- ways and enhanced early mobilization after TKA has driven the scribe their relative contributions to the innervation of the knee joint. search for more peripheral sites of local anesthetic administration Methods: After research ethics board approval, 20 cadaveric lower limbs in an attempt to preserve postoperative quadriceps strength. The were examined using standard dissection technique. Branches of both the adductor canal, also known as the subsartorial or Hunter canal, femoral and obturator nerves were explored along the adductor canal and has been proposed as one such location.6–8 Early data suggest that all branches followed to their termination. -

Species - Domesticus (Chicken) to Mammals (Human Being)
Int. J. LifeSc. Bt & Pharm. Res. 2013 Sunil N Tidke and Sucheta S Tidke, 2013 ISSN 2250-3137 www.ijlbpr.com Vol. 2, No. 4, October 2013 © 2013 IJLBPR. All Rights Reserved Research Paper MORPHOLOGY OF KNEE JOINT - CLASS- AVES - GENUS - GALLUS, - SPECIES - DOMESTICUS (CHICKEN) TO MAMMALS (HUMAN BEING) Sunil N Tidke1* and Sucheta S Tidke2 *Corresponding Author: Sunil N Tidke [email protected] In the present investigation, a detailed comparison is made between the human knee and the knee of chicken (Gallus domesticus), with the object of determining similarities or variation of structure and their possible functional significance, if any special attention has been paid to bone taking part in joint, the surrounding muscles and tendons, which play an important part in stabilizing these joints, the form and attachments of the intraarticular menisci, which have been credited with the function of ensuring efficient lubrication throughout joints movement, and to the ligaments, the function of which is disputed. Keywords: Bony articular part, Intra capsular and extra capsular structure and Muscular changes INTRODUCTION patella. A narrow groove on the lateral condyle of femur articulate with the head of the fibula and The manner in which the main articulations of intervening femoro fibular disc. The tibia has a the vertebrate have become variously modified enormous ridge and crest for the insertion of the in relation to diverse function has been patellar tendon and origin of the extensor muscle. investigated by many workers, notably, Parsons The cavity of the joint communicates above and (1900) and Haines (1942). The morphology of the below the menisci with the central part of joint knee joint of human has been studied in great around the cruciate ligament. -
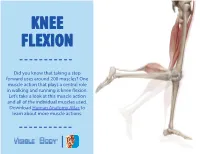
Did You Know That Taking a Step Forward Uses Around 200 Muscles? One Muscle Action That Plays a Central Role in Walking and Running Is Knee Flexion
KNEE FLEXION Did you know that taking a step forward uses around 200 muscles? One muscle action that plays a central role in walking and running is knee flexion. Let’s take a look at this muscle action and all of the individual muscles used. Download Human Anatomy Atlas to learn about more muscle actions. KNEE CAP KNEE MCL ACL OVERVIEW The knee is one of the largest joints in the human body. It provides shock absorption during walking and running, and allows flexion and extension. The knee’s stability is maintained MEDIAL by different ligaments like the MENISCUS anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament LCL PCL LATERAL MENISCUS (MCL), medial meniscus, lateral meniscus, lateral collateral ligament (LCL). ANTERIOR VIEW POSTERIOR VIEW KNEE EXTENSION KNEE FLEXION KNEE FLEXION OVERVIEW Knee flexion is the action of the knee bending the leg towards the buttock. The reverse of this action, when the lower leg is straightened, is called knee extension. Sartorius MUSCLES Gracilis OF Semitendinosus KNEE FLEXION Semimembranosus Biceps Femoris (Long Head) Biceps Femoris (Short Head) There are 8 main muscles used in knee flexion. We will take a look at each one Gastrocnemius and their individual origins, insertions, innervation, and blood supply points. Achilles Tendon SARTORIUS The sartorius is the longest muscle in the body. It is located in the anterior compartment of the thigh and assists in the movements of the hip, thigh, knee, and lower leg. Origin: Ilium (anterior superior iliac spine) Insertion: -

Thigh Muscles
Lecture 14 THIGH MUSCLES ANTERIOR and Medial COMPARTMENT BY Dr Farooq Khan Aurakzai PMC Dated: 03.08.2021 INTRODUCTION What are the muscle compartments? The limbs can be divided into segments. If these segments are cut transversely, it is apparent that they are divided into multiple sections. These are called fascial compartments, and are formed by tough connective tissue septa. Compartments are groupings of muscles, nerves, and blood vessels in your arms and legs. INTRODUCTION to the thigh Muscles The musculature of the thigh can be split into three sections by intermuscular septas in to; Anterior compartment Medial compartment and Posterior compartment. Each compartment has a distinct innervation and function. • The Anterior compartment muscle are the flexors of hip and extensors of knee. • The Medial compartment muscle are adductors of thigh. • The Posterior compartment muscle are extensor of hip and flexors of knee. Anterior Muscles of thigh The muscles in the anterior compartment of the thigh are innervated by the femoral nerve (L2-L4), and as a general rule, act to extend the leg at the knee joint. There are three major muscles in the anterior thigh –: • The pectineus, • Sartorius and • Quadriceps femoris. In addition to these, the end of the iliopsoas muscle passes into the anterior compartment. ANTERIOR COMPARTMENT MUSCLE 1. SARTORIUS Is a long strap like and the most superficial muscle of the thigh descends obliquely Is making one of the tendon of Pes anserinus . In the upper 1/3 of the thigh the med margin of it makes the lat margin of Femoral triangle. Origin: Anterior superior iliac spine. -

Iliopsoas Muscle Injury in Dogs
Revised January 2014 3 CE Credits Iliopsoas Muscle Injury in Dogs Quentin Cabon, DMV, IPSAV Centre Vétérinaire DMV Montréal, Quebec Christian Bolliger, Dr.med.vet, DACVS, DECVS Central Victoria Veterinary Hospital Victoria, British Columbia Abstract: The iliopsoas muscle is formed by the psoas major and iliacus muscles. Due to its length and diameter, the iliopsoas muscle is an important flexor and stabilizer of the hip joint and the vertebral column. Traumatic acute and chronic myopathies of the iliopsoas muscle are commonly diagnosed by digital palpation during the orthopedic examination. Clinical presentations range from gait abnormalities, lameness, and decreased hip joint extension to irreversible fibrotic contracture of the muscle. Rehabilitation of canine patients has to consider the inciting cause, the severity of pathology, and the presence of muscular imbalances. ontrary to human literature, few veterinary articles have been Box 2. Main Functions of the Iliopsoas Muscle published about traumatic iliopsoas muscle pathology.1–6 This is likely due to failure to diagnose the condition and the • Flexion of the hip joint C 5 presence of concomitant orthopedic problems. In our experience, repetitive microtrauma of the iliopsoas muscle in association with • Adduction and external rotation of the femur other orthopedic or neurologic pathologies is the most common • Core stabilization: clinical presentation. —Flexion and stabilization of the lumbar spine when the hindlimb is fixed Understanding applied anatomy is critical in diagnosing mus- —Caudal traction on the trunk when the hindlimb is in extension cular problems in canine patients (BOX 1 and BOX 2; FIGURE 1 and FIGURE 2). Pathophysiology of Muscular Injuries Box 1.