Exploring Topical Anti-Glaucoma Medication Effects on the Ocular Surface in the Context of the Current Understanding of Dry Eye
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Challenges in Ophthalmic Pathology: the Vitreoretinal Membrane Biopsy
Challenges in PAUL HISCOTT, DAVID WONG, IAN GRIERSON ophthalmic pathology: The vitreoretinal membrane biopsy Abstract detachment.s Sheets or strands crossing the vitreous are sometimes called transvitreous The introduction of vitreoretinal microsurgery membranes. Anteriorly, membranes can arise has produced a new type of biopsy; that of the in, or be continuous with, the vitreous base and vitreoretinal membrane. This review even extend as far as the posterior iris surface or investigates methods by which these scar-like pupil. tissues are handled in the laboratory and Biopsies of pathological tissue are usually explores the implications of the results of such undertaken to establish a diagnosis. evaluations. The study of vitreoretinal Vitreoretinal biopsies also may be for diagnostic membrane biopsies has provided much purposes as, for example, in the case of information concerning the pathobiology of intraocular lymphoma, but such conditions are the various conditions which may give rise to rare and tend not to produce membranes. the tissue as well as insights into how Conversely, in the conditions which do produce membranes themselves develop. Moreover, membranes the diagnosis is seldom in doubt. the application of new laboratory techniques Why, then, attempt laboratory studies of is expected to enhance our understanding of vitreoretinal membranes? Laboratory findings the formation of vitreoretinal membranes, and from the membranes may have a number of lead to further advances in their surgical and uses, for example providing 'feed-back' to the medical management. surgeon concerning surgical dissection planes Key words Age-related macular degeneration, (see below). However, the principal objective of Epiretinal membrane, Proliferative diabetic these investigations is to improve our retinopathy, Proliferative vitreoretinopathy, understanding of the pathogenesis of P Hiscott D. -

SHOULD YOU OPEN a DRY-EYE CLINIC? Experts Help You Weigh the Pros and Cons
RETINAL PHOTOS ON THE GO P. 16 • CHANGES TO MEDICARE ABN FORMS P. 20 ENSURING A HAPPY CATARACT PATIENT P. 22 • MANAGING GLAUCOMA IN KPRO PATIENTS P. 58 THE CURRENT STATE OF VITREORETINAL EDUCATION P. 64 • WILLS EYE RESIDENT CASE REPORT P. 70 September 2020 reviewofophthalmology.com SHOULD YOU OPEN A DRY-EYE CLINIC? Experts help you weigh the pros and cons. P. 28 ALSO INSIDE: • The Latest Treatments for Dry Eye P. 38 • Comprehensive Ophthalmologists and Anti-VEGF Injections P. 48 • How to Manage Ocular Herpes P. 54 SHE MAY NEED MORE THAN ARTIFICIAL TEARS TO DISRUPT INFLAMMATION IN DRY EYE DISEASE1,2 Her eyes deserve a change. Choose twice-daily Xiidra for lasting relief that can start as early as 2 weeks.3*† Not an actual patient. *In some patients with continued daily use. One drop in each eye, twice daily (approximately 12 hours apart).3 †XiidraisanLFA-1antagonistforthetreatmentofdryeyedisease.Pivotaltrialdata:ThesafetyandefficacyofXiidrawereassessedinfour Important Safety Information (cont) 12-week,randomized,multicenter,double-masked,vehicle-controlledstudies(N=2133).Patientsweredosedtwicedaily.Useofartificial • I n clinicaltrials,themostcommonadversereactionsreportedin5-25%ofpatientswereinstillationsite tearswasnotallowedduringthestudies.Thestudyendpointsincludedassessmentofsigns(basedonInferiorfluoresceinCornealStaining irritation,dysgeusiaandreducedvisualacuity.Otheradversereactionsreportedin1%to5%ofthepatients Score [ICSS] on a scale of 0 to 4) and symptoms (based on patient-reported Eye Dryness Score [EDS] on a visual analogue scale of 0 to 100).3 were blurred vision, conjunctival hyperemia, eye irritation, headache, increased lacrimation, eye discharge, A larger reduction in EDS favoring Xiidra was observed in all studies at day 42 and day 84. Xiidra reduced symptoms of eye dryness at eye discomfort, eye pruritus and sinusitis. 2 weeks (based on EDS) compared to vehicle in 2 out of 4 clinical trials. -

Macular Hole Formation Following Choroidal Neovascularization
Yoshida et al., Int J Ophthalmol Clin Res 2015, 2:1 International Journal of ISSN: 2378-346X Ophthalmology and Clinical Research Case Report: Open Access Macular Hole Formation Following Choroidal Neovascularization Treatment in A High Myopic Eye with Epiretinal Membrane Yusaku Yoshida*, Manabu Yamamoto, Takeya Kohno and Kunihiko Shiraki Department of Ophthalmology and Visual Science, Osaka City University Graduate School of Medicine, Japan *Corresponding author: Yusaku Yoshida, MD, Department of Ophthalmology and Visual Science, Osaka City University Graduate School of Medicine, 1-4-3 Asahi-machi, Abeno-ku, Osaka 545-8585, Japan, Fax:+81-666-343- 873, E-mail: [email protected] macular hole development where post-treatment regression of CNV Abstract seemed to have played a major role in a high myopic eye with ERM. Purpose: To report on the development of macular hole after treatment of choroidal neovascularization (CNV) in a patient with Case high myopia and epiretinal membrane (ERM). A 60-year-old man was examined in the Department of Methods: Retrospective single case report. Ophthalmology of Osaka City University Hospital for reduced vision Patients: A 60-year-old man presented with reduced vision in his in his right eye in January 2009. On initial examination, decimal best- right eye. On the initial examination, the right eye was high myopic corrected visual acuity (BCVA) was 0.3 in the right eye and 1.5 in with decimal best-corrected visual acuity (BCVA) of 0.3. the left eye, while intraocular pressures were 13 and 14 mmHg in the right and left eye, respectively. His previous medical and family Results: A subretinal grayish-white lesion was seen at the macula with hemorrhage, and optical coherence tomography showed a histories included nothing of note. -

Epiretinal Membrane
PATIENT INFORMATION LEAFLET British and Eire Association of Vitreoretinal Surgeons EPIRETINAL MEMBRANE What is an Epiretinal Membrane? An Epiretinal Membrane is a condition where a very thin layer of scar tissue forms on the surface of the retina, where the vision is sharpest. The part of the eye affected by the Epiretinal Membrane is called the Macula, which is made of special nerve cells and it provides our sharp central vision needed for seeing fine detail (reading and driving etc.). When an Epiretinal Membrane forms over the Macula, it may contract and crumple up the Macula resulting in distorted and/or blurred vision. The normal anatomy of the eye *Image courtesy of the National Eye Institute http://www.nei.nih.gov Page 1 of 5 PATIENT INFORMATION British and Eire Association of Vitreoretinal Surgeons LEAFLET EPIRETINAL MEMBRANE Why do I have an Epiretinal Membrane? In most cases the development of an Epiretinal Membrane appears to be related to normal aging changes inside the eye. In some cases it can be related to other conditions such as diabetes, blockage of blood vessel, inflammation or following retinal surgery. Epiretinal membranes are not related to Macular Degeneration. Epiretinal Membranes do not usually affect the other eye. They are quite common and affect up to 8% of people in later years. Cross-section of a healthy macula Cross-section of a macula with an epiretinal membrane Assessment for Epiretinal Membrane Your eye doctor is able to detect an Epiretinal Membrane during an eye examination following the use of eye drops that temporarily make your pupils large. -

Home>>Common Retinal & Ophthalmic Disorders
Common Retinal & Ophthalmic Disorders Cataract Central Serous Retinopathy Cystoid Macular Edema (Retinal Swelling) Diabetic Retinopathy Floaters Glaucoma Macular degeneration Macular Hole Macular Pucker - Epiretinal Membrane Neovascular Glaucoma Nevi and Pigmented Lesions of the Choroid Posterior Vitreous Detachment Proliferative Vitreoretinopathy (PVR) Retinal Tear and Detachment Retinal Artery and Vein Occlusion Retinitis Uveitis (Ocular Inflammation) White Dot Syndromes Anatomy and Function of the Eye (Short course in physiology of vision) Cataract Overview Any lack of clarity in the natural lens of the eye is called a cataract. In time, all of us develop cataracts. One experiences blurred vision in one or both eyes – and this cloudiness cannot be corrected with glasses or contact lens. Cataracts are frequent in seniors and can variably disturb reading and driving. Figure 1: Mature cataract: complete opacification of the lens. Cause Most cataracts are age-related. Diabetes is the most common predisposing condition. Excessive sun exposure also contributes to lens opacity. Less frequent causes include trauma, drugs (eg, systemic steroids), birth defects, neonatal infection and genetic/metabolic abnormalities. Natural History Age-related cataracts generally progress slowly. There is no known eye-drop, vitamin or drug to retard or reverse the condition. Treatment Surgery is the only option. Eye surgeons will perform cataract extraction when there is a functional deficit – some impairment of lifestyle of concern to the patient. Central Serous Retinopathy (CSR) Overview Central serous retinopathy is a condition in which a blister of clear fluid collects beneath the macula to cause acute visual blurring and distortion (Figure 2). Central serous retinochoroidopathy Left: Accumulation of clear fluid beneath the retina. -
Retinal Conditions
GENERAL INFORMATION RETINAL CONDITIONS RETINAL CONDITIONS WHAT ARE RETINAL CONDITIONS? Retinal conditions affect the light-sensitive tissue at the back of eye known as the retina. They include diseases that affect the part of the retina responsible for central vision (the macula) and the jelly-like fluid that sits in front of the retina (the vitreous). The retina acts like the ‘film’ of a camera, sending information about shapes, colours, patterns and movement via the optic nerve to the brain, where the information is processed into the final image that we see. DAMAGE TO THE RETINA CAUSES VISUAL DISTURBANCES OR LOSS OF VISION. RETINAL CONDITIONS 2 RETINAL CONDITIONS The main conditions that affect the retina are: Macular degeneration: A group of degenerative diseases of the macula that cause loss of central and fine-detail vision. Treatment to stop or slow the progression of the wet form includes eye injections and photodynamic therapy. Unfortunately, there is no approved treatment for the dry form. Diabetic retinopathy: An eye disease caused by the persistently high blood sugar levels that occur with diabetes. Depending on the stage of disease, treatment includes eye injections, retinal laser treatment and vitrectomy surgery (keyhole surgery to remove the vitreous). The most advanced stage of the disease is an ocular emergency that can cause total vision loss. Retinal vein occlusion: Vision loss that occurs when the veins in the retina are blocked and the build-up of pressure causes smaller downstream blood vessels to leak. The blockage can occur in a branch vein with vision loss restricted to part of the visual field (branch vein retinal occlusion) or a central vein with the entire visual field affected (central vein retinal occlusion). -

Keratoconus and Cone-Rod Dystrophy Among Brothers: Clinical Case Study and Genetic Analysis
Trends in Ophthalmology Open Access Journal DOI: 10.32474/TOOAJ.2019.02.000133 ISSN: 2644-1209 Case Report Keratoconus and Cone-Rod Dystrophy among Brothers: Clinical Case Study and Genetic Analysis Victoria Dimacali1*, Spyridon Koronis1, Stavrenia Koukoula1, Achilleas Rasoglou2, Aspasia Adamopoulou1, Elissavet Siskou3 and Miltos Balidis1 1Ophthalmica Institute of Ophthalmology and Microsurgery, Greece 2Department of Ophthalmology, General Hospital of Edessa, Greece 3Department of Ophthalmology, Papanikolaou General Hospital, Greece *Corresponding author: Victoria Dimacali, Ophthalmica Institute of Ophthalmology and Microsurgery, Thessaloniki, Greece Received: March 25, 2019 Published: April 01, 2019 Abstract Background: Advances in genomics continue to enable the discovery of gene variants which cause various inherited ophthalmic disorders. Several case reports have shown an association between keratoconus and retinal disease but whether there is a genetic basis for this is still not known. Methods: Clinical case study with Pentacam imaging, fundus autofluorescence (FAF), macular optical coherence tomography (OCT),Results: electrophysiology We report three studies, brothers, and genetic two of whomanalysis. have keratoconus and one who was found to have bilateral cone-rod dystrophy. MAP3K19, ADGRV1, and PIK3CG This was supported by color vision and electrophysiology testing, fundus autofluorescence,CHST6 gene in and the macular latter. Whole OCT exomefindings. sequencing Genomic data revealedanalysis revealeda rare missense three rare variant gene for variants IMPG2 ( gene in both brothers. ) common to the brother with cone-rod dystrophy and one brother with keratoconus. There was also a very significant variant in the Conclusion: Among the four genes with shared mutations in two of the brothers, IMPG2 has been linked to retinal disease while MAP3K19 and PIK3CG carry high risk scores for keratoconus pathogenesis. -

Clinical Study Epiretinal Membrane Surgery in Daily Clinical Practice: Results of a Proposed Management Scheme
Hindawi Journal of Ophthalmology Volume 2019, Article ID 8246858, 6 pages https://doi.org/10.1155/2019/8246858 Clinical Study Epiretinal Membrane Surgery in Daily Clinical Practice: Results of a Proposed Management Scheme Jesu´ s Pareja,1,2 Alba Coronado,1 and Ine´s Contreras 1,3 1Cl´ınica Rementer´ıa, Madrid, Spain 2Instituto Provincial de Oftalmolog´ıa-HGU Gregorio Maraño´n, Madrid, Spain 3Hospital Universitario Ram´on y Cajal. Instituto Ram´on y Cajal de Investigaciones Sanitarias (IRYCIS), Madrid, Spain Correspondence should be addressed to In´es Contreras; [email protected] Received 16 October 2018; Accepted 23 December 2018; Published 10 January 2019 Academic Editor: Elad Moisseiev Copyright © 2019 Jes´us Pareja et al. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. To report the results of the epiretinal membrane (ERM) management guidelines followed in our center. Methods. Patients with ERM seen between 2014 and 2015, with ≥2 years follow-up or who had undergone ERM surgery, were included. Corrected visual acuity (VA), lens status, and ERM configuration were recorded at each visit. Our guidelines for ERM are if VA is ≥20/30, observation is recommended unless there is moderate/intense metamorphopsia. Vitrectomy is recommended during follow-up if there is a drop >one line in VA with changes in ERM configuration. If VA at diagnosis is <20/30, vitrectomy is recommended. If visual loss is thought to be due to cataract, phacoemulsification is performed first and visual status reevaluated. -

Epiretinal Membrane Formation Following Rhegmatogenous Retinal Detachment Repair: Optical Coherence Tomography Features and Surgical Outcomes
Epiretinal Membrane Formation Following Rhegmatogenous Retinal Detachment Repair: Optical Coherence Tomography Features and Surgical Outcomes M. Ali Khan, MD Retina Service, Wills Eye Hospital Assistant Professor, Kimmel Medical College Philadelphia, PA Retina Society Annual Meeting, 2020 Financial Disclosures • No relevant financial disclosures. • Consulting fees outside the submitted work: Allergan, Apellis Conclusions • Anatomic alteration due to epiretinal membrane (ERM) formation after RRD repair is commonly severe (Stage 4 OCT characteristics), and leads to significant worsening of logMAR visual acuity. • ERM removal with vitrectomy and membrane peeling resulted in a significant improvement in visual acuity in eyes with history of either macula on or macula off RRD. • Ectopic inner foveal layer (EIFL) thickness, IS/OS disruption, and microcytic changes were associated with visual acuity at 6 months post ERM peel using regression analysis. Confirmation of these associations in larger series is warranted. Epiretinal membrane after RRD Repair • Epiretinal membrane (ERM) formation after primary rhegmatogenous retinal detachment (RRD) repair is common, with estimates ranging from 4-13%.[1-4] • Several authors have described the utility of internal limiting membrane (ILM) peel during primary RRD repair to prevent later ERM formation.[5-9] • Preservation of IS/OS band on SD-OCT has been correlated to visual acuity improvement after membrane peel surgery.[10] • Macular status at time of RRD repair may determine visual potential. OCT Grading of ERM • Govetto et al. described a new, SD-OCT based grading scheme for ERM in 2017.[11,12] Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme ANDREA GOVETTO, ROBERT A. -

Toxic Anterior Segment Syndrome After an Uncomplicated Vitrectomy with Epiretinal Membrane Peeling
Open Access Case Report DOI: 10.7759/cureus.14464 Toxic Anterior Segment Syndrome After an Uncomplicated Vitrectomy With Epiretinal Membrane Peeling Piotr Kanclerz 1 1. Ophthalmology, Hygeia Clinic, Gdansk, POL Corresponding author: Piotr Kanclerz, [email protected] Abstract Infectious endophthalmitis is the most devastating complication of eye surgery and is associated with severe inflammation of ocular tissues. This study aimed to present a similar condition, a case of toxic anterior segment syndrome (TASS) after an uncomplicated vitrectomy. A 69-year-old woman presented with epiretinal membrane and underwent 25-gauge pars plana vitrectomy with membrane peeling in her left eye. Thirty hours after the procedure, the patient complained of increasing loss of visual acuity and a red left eye. The ophthalmic examination revealed moderate hyperemia, hypopyon and snowbanks in the anterior vitreous. Subconjunctival and topical steroids were administered, and the inflammatory symptoms resolved within 30 days. The visual acuity improved to 20/32, however, cystoid changes were noted in the macula by optical coherence tomography. TASS should be considered a potential complication after vitrectomy. This report presents a case of TASS and discusses the differential diagnosis between TASS, infectious and non-infectious endophthalmitis. Categories: Ophthalmology Keywords: endophthalmitis, epiretinal membrane, toxic anterior segment syndrome, pars planitis, vitrectomy Introduction Postoperative endophthalmitis (POE) is the most devastating complication of intraocular surgery, and is associated with severe inflammation of ocular issues. POE following vitrectomy is relatively uncommon; in large studies the incidence rates range between 0.02% and 0.15% [1]. Toxic anterior segment syndrome (TASS) is defined as a sterile postoperative inflammation of the anterior segment after intraocular surgery [2]. -

Spontaneous Peeling of Epiretinal Membrane Associated with Nd:YAG Laser Injury
CASE REPORTS AND SMALL CASE SERIES An Ocular Endoscope Enables a Goniotomy Despite a Cloudy Cornea Infantile glaucoma is often initially treated with a surgical goniotomy or trabeculotomy. A goniotomy is not possible if the cornea is too cloudy, despite preoperative glaucoma medi- cations and removal of the corneal epithelium. Bimanual endoscopic goniotomy has been reported in 1 child, but this technique requires great dexterity to maintain the en- doscopic image on the needle tip.1 In the following case, we used a new technique, coaxial endoscopic go- A niotomy,2 that allowed a goni- otomy to be performed when the an- terior chamber angle could not be distinguished through the surgical gonioprism. Report of a Case. A 19-month-old girl was referred with a several- month history of film covering both eyes. The child was photophobic, tearing, and had bilateral buphthal- mos with extremely cloudy cor- neas. She was prescribed timolol ma- leate, latanoprost, and acetazolamide sodium syrup while undergoing treatment for otitis media. She had no other health problems. An ex- amination under anesthesia 9 days later revealed intraocular pressures B of 31 mm Hg OD and 33 mm Hg OS, corneal diameters of 14.5 mm OD Figure 1. Images are from surgical videotapes. A, Structures in the anterior chamber angle in the left eye and 15 mm OS, circumferential and were not clearly visualized through a surgical gonioprism. A Haab stria is also present (arrow). B, The anterior chamber angle that was treated by coaxial endoscopic goniotomy is viewed several weeks later horizontal Haab striae in both eyes, through a surgical gonioprism. -
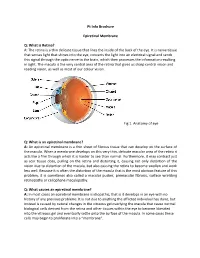
Epiretinal Membrane
Pt Info Brochure Epiretinal Membrane Q: What is Retina? A: The retina is a thin delicate tissue that lines the inside of the back of the eye. It is nerve tissue that senses light that shines into the eye, converts the light into an electrical signal and sends this signal through the optic nerve to the brain, which then processes the information resulting in sight. The macula is the very central area of the retina that gives us sharp central vision and reading vision, as well as most of our colour vision. Fig 1. Anatomy of eye Q: What is an epiretinal membrane? A: An epiretinal membrane is a thin sheet of fibrous tissue that can develop on the surface of the macula. When a membrane develops on this very thin, delicate macular area of the retina it acts like a film through which it is harder to see than normal. Furthermore, it may contract just as scar tissue does, pulling on the retina and distorting it, causing not only distortion of the vision due to distortion of the macula, but also causing the retina to become swollen and work less well. Because it is often the distortion of the macula that is the most obvious feature of this problem, it is sometimes also called a macular pucker, premacular fibrosis, surface wrinkling retinopathy or cellophane maculopathy. Q: What causes an epiretinal membrane? A: In most cases an epiretinal membrane is idiopathic, that is it develops in an eye with no history of any previous problems. It is not due to anything the afflicted individual has done, but instead is caused by natural changes in the vitreous gel overlying the macula that cause normal biological cells derived from the retina and other tissues within the eye to become liberated into the vitreous gel and eventually settle onto the surface of the macula.