Finite Element Simulation of Skull Fracture Evoked by Fall Injuries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of Occurrence and Types of Suprameatal Spines in the Suprameatal Triangle
ISSN: 2455-2631 © March 2021 IJSDR | Volume 6, Issue 3 A STUDY OF OCCURRENCE AND TYPES OF SUPRAMEATAL SPINES IN THE SUPRAMEATAL TRIANGLE Preetha Parthasarathy Under graduate SIMATS Saveetha Dental College, 162, Poonamalle high road, Velappanchavadi, Chennai- 600095. India. Mrs. M.S. Thenmozhi Dept of Anatomy SIMATS Saveetha Dental College, 162, Poonamalle high road, Velappanchavadi, Chennai- 600095. India. Corresponding author: Mrs. Thenmozhi. M.S Dept of anatomy SIMATS Saveetha dental college, Velappanchavadi Chennai-600095 India Running title: Types of suprameatal spines ABSTRACT: AIM: To identify the occurrence and types of suprameatal spines on either sides of the skull in the suprameatal triangle and to study their clinic implications. OBJECTIVE: To figure out the presence of suprameatal spines in the suprameatal triangle and to review the literature on anatomical and clinic aspects of suprameatal triangle. INTRODUCTION: Suprameatal triangle is present between the posterior wall of external acoustic meatus and posterior root of zygomatic process, in the temporal bone. It is also called as Macewen’s triangle. Suprameatal spine is seen below the upper limit of the orifice of the inner end of external acoustic meatus which is closed by tympanic membrane. It is also called as spine of Henle. RESULT: From the above conducted study, it can be seen that, when the suprameatal spines were evaluated according to its type and occurrence, the crest type of spine present was more than the triangle type. The crest type of spine was found to be 62.2% and the triangle type of spine was about 37.8% CONCLUSION: Thus, this study shows the prevalence of crest and triangle type spine in the dry skulls evaluated. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Macewen'striangle
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 5, 2020 MacEwen’sTriangle- A Review Dr. Bhaskaran Sathyapriya, Professor, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai Chandrakala B1, Govindarajan Sumathy2, Syed FazilHasan 3, Priyadharshini.M3, Srilakshmi.B 3,Bhaskaran Sathyapriya* 1. Senior Lecturer, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. 2. Professor and Head, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. 3. Graduate student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research *Professor, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. Abstract In the temporal bone, between the posterior wall of the external acoustic meatus and the posterior root of the zygomatic process is the area called the suprameatal triangle, suprameatal pit, mastoid fossa, foveolasuprameatica, or Mac Ewen's triangle, through which an instrument may be pushed into the mastoid antrum..In the adult, the antrum lies approximately 1.5 to 2 cm deep to the suprameatal triangle. This is an important landmark when performing a cortical mastoidectomy. The triangle lies deep to the cymba conchae.The sex determination of unknown human skulls can be evaluated by using the measurement of the area formed by the xerographic projection of 3craniometric points related to the mastoid process: the porion, asterion, and mastoidale points. Keywords: MacEwen's triangle,mastoidectomy,suprameatal spine,Mastoid antrum ,sex determination,zygomatic process,mastoid process. Introduction MacEwen's triangle is a very important surgical landmark for the mastoid antrum or the largest mastoid air cell.[9] It is also known as Suprameatal triangle or Mastoid fossa.[4] The suprameataltrigone plays a big role in the aspect of clinics. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Modern Surgery, 4Th Edition, by John Chalmers Da Costa Rare Medical Books
Thomas Jefferson University Jefferson Digital Commons Modern Surgery, 4th edition, by John Chalmers Da Costa Rare Medical Books 1903 Modern Surgery - Chapter 23. Diseases and Injuries of the Head John Chalmers Da Costa Jefferson Medical College Follow this and additional works at: https://jdc.jefferson.edu/dacosta_modernsurgery Part of the History of Science, Technology, and Medicine Commons Let us know how access to this document benefits ouy Recommended Citation Da Costa, John Chalmers, "Modern Surgery - Chapter 23. Diseases and Injuries of the Head" (1903). Modern Surgery, 4th edition, by John Chalmers Da Costa. Paper 29. https://jdc.jefferson.edu/dacosta_modernsurgery/29 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Modern Surgery, 4th edition, by John Chalmers Da Costa by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Diseases of the Head 595 XXIII. DISEASES AND INJURIES OF THE HEAD. I. DISEASES OF I:: HEAD. IN approaching a case of brain disorder, first endeavor to locate the seat of the trouble; next, ascertain the nature of the lesion; and, finally, deter- mine the best plan of treatment, operative or otherwise. -

Inferior View of Skull
human anatomy 2016 lecture sixth Dr meethak ali ahmed neurosurgeon Inferior View Of Skull the anterior part of this aspect of skull is seen to be formed by the hard palate.The palatal process of the maxilla and horizontal plate of the palatine bones can be identified . in the midline anteriorly is the incisive fossa & foramen . posterolaterlly are greater & lesser palatine foramena. Above the posterior edge of the hard palate are the choanae(posterior nasal apertures ) . these are separated from each other by the posterior margin of the vomer & bounded laterally by the medial pterygoid plate of sphenoid bone . the inferior end of the medial pterygoid plate is prolonged as a curved spike of bone , the pterygoid hamulus. the superior end widens to form the scaphoid fossa . posterolateral to the lateral pterygoid plate the greater wing of the sphenoid is pieced by the large foramen ovale & small foramen spinosum . posterolateral to the foramen spinosum is spine of the sphenoid . Above the medial border of the scaphoid fossa , the sphenoid bone is pierced by pterygoid canal . Behind the spine of the sphenoid , in the interval between the greater wing of the sphenoid and the petrous part of the temporal bone , there is agroove for the cartilaginous part of the auditory tube. The opening of the bony part of the tube can be identified. The mandibular fossa of the temporal bone & the articular tubercle form the upper articular surfaces for the temporomandibular joint . separating the mandibular fossa from the tympanic plate posteriorly is the squamotympanic fissure, through the medial end of which (petrotympanic fissure ) the chorda tympani exits from the tympanic cavity .The styloid process of the temporal bone projects downward & forward from its inferior aspect. -
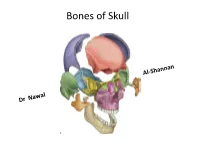
Norma Lateralis: Lateral View of the Skull • Bones; • 1.Temporal Bone • 2.Temporal Lines • 3Zygomatic Arch • 4.External Auditory Meatus • 5
Bones of Skull Views of the skull Each aspect of skull is called Norma : study from above is called Norma verticalis Below : Norma - basalis Behind : Norma - occipitalis Front : Norma - frontalis Side to side: Norma - lateralis Norma verticalis = Vault = arched roof = dome • Bones: • Frontal • Parietal • Small part of occipital • Vertex : the highest point of the sagittal suture • Paraietal foramen – 3.5 cm infront of lambda • Bregma: cranial point between coronal and sagittal suture • Lambda: cranial point at the junction of lambda and sagittal suture • Paraietal eminence: maximum convex part of parietal bone Vault Norma occipitalis: posterior aspect of the skull • Bones: • 1. Posterior part of parietal bone • 2.mastoid process of temporal bone • 3.squamous part of occipital bone • Sutures: • 1.lambdoid • Paraietomastoid • occipitomastoid • External occipital protuberance • Superior and highest nuchal line Posterior View &Sutures Norma Frontalis • Can be divided into upper and lower Upper part: Super ciliary arches: curved elevations above the supraorbital margins Glabella: median elevation between the two supraciliary arches Nasion: median point at the root of the nose oribital opening : supraorbital margin formed by frontal bone supraorbital notch or foramen transmit supraorbital vessels and nerves Infra orbital margin formed by maxilla medially and zygomatic bone laterally Medial margin: formed by frontal bone above and anterior lacrimal crest of the maxilla below Lateral margin; formed by frontal process of zygomatic bone and zygomatic process of frontal bone Anterior nasal aperture Nasal bones Norma frontalis Upper part / Orbit Upper part Norma frontalis lower part of the face: Maxilla have processess Frontal process of maxilla Zygomatic process Alveolar process Zygomatic bones Body ,processes ,arch Zygomaticofacial foramen Z-f nerve Mandible : mental f -mental n and v Lower part Norma lateralis: lateral view of the skull • Bones; • 1.temporal bone • 2.temporal lines • 3Zygomatic arch • 4.External auditory meatus • 5. -

Skull Joints
Svintsytska N.L. Hryn V.H. Kovalchuk O.І. MORPHOFUNCTIONAL CHARACTERISTIC OF THE SKULL WITH A CLINICAL ASPECT Study guide Poltava 2020 MINISTRY OF PUBLIC HEALTH OF UKRAINE UKRANIAN MEDICAL STOMATOLOGICAL ACADEMY Nataliya L. Svintsytska, Volodymyr H. Hryn, Oleksandr I. Kovalchuk MORPHOFUNCTIONAL CHARACTERISTIC OF THE SKULL WITH A CLINICAL ASPECT Study guide Poltava 2020 2 UDC 616.714:612 "Recommended by the Academic Council of the Ukrainian Medical Stomatological Academy as a study guide for foreign students receiving higher master's degrees, studying in the specialties 221"Stomatology", 222" Medicine "in higher educational institutions of of the Ministry of Health of Ukraine." Minutes of the meeting of the Academic Council of the Ukrainian Medical Stomatological Academy from 11.03.2020 №8. Composed by: Svintsytska N.L., Associate Professor at the Department of Human Anatomy of Ukrainian Medical Stomatological Academy, PhD in Medicine, Associate Professor Hryn V.H., Associate Professor at the Department of Human Anatomy of Ukrainian Medical Stomatological Academy, PhD in Medicine, Associate Professor Kovalchuk O.І., Head of the Department of Аnatomy and Pathological Physiology of Educational and Scientific Center "Institute of Biology and Medicine" of Taras Shevchenko National University of Kyiv, Doctor of Medical Sciences, Professor Study guide for foreign students of higher medical educational institutions оf Ukraine in Specialties – 222 «Medicine» та 221 «Stomatology». Morphofunctional characteristic of the skull with a clinical aspect. – Poltava, 2020 – 205 р. This guide is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. -

Scientific Article
SCIENTIFIC ARTICLE Anatomical landmarks to locate the junction between transverse and sigmoid sinuses in translabyrinthine and retrosigmoid open surgical approaches Y Mathangasinghe1, UMJE Samaranayake1, SSA Silva1, HC Prematilaka1, MHS Perera1, SSW Shantha2 1 Department of Anatomy, Faculty of Medicine, University of Colombo, Colombo, Sri Lanka 2 Neville Fernando Teaching Hospital, Malabe, Sri Lanka Keywords: Transverse sinus; sigmoid sinus; translaby- Discussion and conclusions rinthine approach; retrosigmoid approach; cerebellopontine Suprameatal triangle was a consistent surface landmark to angle locate dural-sinuses. Dural-sinus damage could be avoided in 95% of the times by placing burr hole at least 7mm superior Abstract and 23 mm posterior to the suprameatal triangle. Introduction Hematoma due to dural-sinus damage is a known Introduction complication when introducing burr holes in open trans- Cerebellopontine angle (CPA) approach during neurosurgery cranial surgery. Our objective was to identify safe areas to is challenging (1). CPA is located posterior to the petrous part avoid dural-sinus damage based on anatomical landmarks in of temporal bone, anterior to the frontal part of cerebellum, translabyrinthine and retrosigmoid open surgical approaches superior to the arachnoid tissue of lower cranial nerves (1). where neuronavigation facilities are not available. CPA is a frequent site of neoplasms and vascular anomalies (2). The commonest pathology that requires surgical Methods resection in this area is the CPA tumors, which has an A descriptive anatomical study was conducted on adult incidence of 4% (2, 3). Acoustic schwannomas are benign skulls. Distances to transverse and sigmoid sinuses on either tumors accounting for approximately 80% of tumors of the side were measured using fixed anatomical landmarks: CPA (4). -

FIPAT-TA2-Part-2.Pdf
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART II Contents: Systemata musculoskeletalia Musculoskeletal systems Caput II: Ossa Chapter 2: Bones Caput III: Juncturae Chapter 3: Joints Caput IV: Systema musculare Chapter 4: Muscular system Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput II: OSSA Chapter 2: BONES Latin term Latin synonym UK English US English English synonym Other 351 Systemata Musculoskeletal Musculoskeletal musculoskeletalia systems systems -

Book Jan-March 2021.Indb
640 Indian Journal of Forensic Medicine & Toxicology, January-March 2021, Vol. 15, No. 1 Morphometric study of Macewan’s Triangle in Relation to Depth of the Sigmoid Sinus Plate Senthil Kumar. B Assistant Professor in Anatomy / Head – Central Research Laboratory for Biomedical Research, Vinayaka Mission’s Kirupananda Variyar Medical College and Hospital, Vinayaka Mission’s Research Foundation (Deemed to be University) Salem, TamilNadu, India. Abstract Background: The suprameatal triangle is used for approaching the tympanic cavity and also it is an important landmark for otologic surgeons during mastoidectomy. Aim: To determine the depth of sigmoid sinus plate over the Macewan’s triangle by morphometry of various anatomical landmarks. Materials & Methods: The study was carried out in the Department of Anatomy, Penang International Dental College & VMKV Medical College, Salem on 30 dry human adult skulls. Five landmarks (D1, D2, D3, D4, D5 and D6) were taken on the left and right sides of the skulls and the imaginary lines were constructed and measured by Vernier caliper between the landmarks. Results: The measurement of D2 showed statistically significant differences. The correlations of D1, D3, D4, D5 and D6 on both sides do not show any significant differences. The linear correlation equations were derived using the measurements for predicting D6. Conclusion: The depth of sigmoid sinus plate (D6) can be assessed using D1, D2, D3, D4, D5 on lateral view of head & neck X-rays which can be used by Otologist during mastoidectomy to avoid severe bleeding complications from sigmois sinus. Keywords - Henle’s spine, Suprameatal triangle, Mastoidectomy, Sigmoid sinus, Introduction the matoid antrum of the mastoid process. -

Clinical Anatomy
Clinical Anatomy, Physiology and Examination of the External and Middle Ear EXTERNAL EAR The external ear includes the auricle and the external auditory meatus. The auricle has a complex configuration and is subdivided into two parts: the lobule, which is a skin duplicature containing adipose tissue, and the skin-covered cartilage. The skin on the posterior surface of the auricle can be folded, while on the anterior surface it adheres tightly to the perichondrium. Several projections are found on the auricle, namely, the helix, the antihelix, the tragus, and the antitragns. The tragus projects anterior to the external auditory meatus (Fig. 27). Pressure on the tragus causes pain in acute otitis externa and also in acute otitis media in infants because their external auditory meatus lacks the osseous part and is therefore shorter than in adults. Applying pressure on. the tragus actually means pressure on the inflamed tympanic membrane, which is necessary for a more accurate location of abnormality in this area (haematoma of the triangular fossa, lobular abscess, etc.). Fig. 27. Auricle 1-helix; 2-entry to the exteraal auditory meatus; 3-antihelix; 4-tragus; 5-lobule; 6-antitragus The normal height of the auricle corresponds to the length of the nasal bridge. Deviations from this norm to either side are microtia or macrotia. The auricle stands out prominently from the head and has a specific blood supply (the vessels of the anterior auricular surface lack a protective layer of fat). Therefore, when exposed to cold, vascular spasm develops, and the ear can easily be affected by frostbite. The auricle plays on important role in ototopia (the ability to locate a sound source) and performs a protective function.