2Fde87e0691f6f570e2a63cb3c9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CST-On-Demand-Binder.Pdf
Zander Perioperative Education Zander CST Exam Preparation Course Zander Perioperative Education Certification Preparation for CNOR, CAPA-CPAN, CST and CBSPD Wendy Zander MSN/Ed, RN, CNOR [email protected] Test Taking Strategies Objectives: 1. Apply Test Taking Strategies for the CST exam 2. Create a Personal Study Plan 3. Eligibility • Registering for the exam • Exam Format • Time Management • Test Taking Strategies Eligibility • Current or previously Certified Surgical Technologist (CST) ▫ Evidence of CST Certification • Graduate of a surgical technology program accredited by CAAHEP ▫ Evidence of proof of graduation • Graduate of a surgical technology accredited by ABHES ▫ Evidence of proof of graduation www.periop-ed.com 1 Zander Perioperative Education Military Eligible • A graduate of a military training program in surgical technology is always eligible whether it was before, during or after having CAAHEP accreditation. ▫ a copy of your DD214 (must state location of the base where program was completed), ▫ a copy of your graduation certificate from the surgical technology training program ▫ a smart transcript Accelerated Alternate Delivery (AAD) Pathway • Have on-the-job training in surgical technology • Are a graduate from a surgical technology program that did not hold CAAHEP accreditation during your enrollment CST Testing Fees First Time Test Takers Exam Fee (AST Members) Exam Fee (Non Members) $190 $290 Current or Previous Certified Surgical Technologist Renewing First Time Test Takers Certification by Examination Exam Fee -
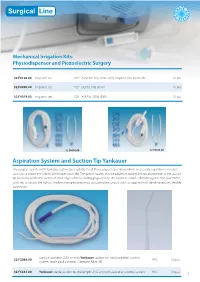
Aspiration System and Suction Tip Yankauer
ILE R S E T E T R S I E L Mechanical Irrigation Kits: Physiodispenser and Piezoelectric Surgery 32.F0106.00 Irrigation set 1/2Y Aseptico Aeu 1000 -70/V, Aseptico Aeu 1070-70V 10 pcs 32.F0090.00 Irrigation set 1/2Y Dental Unit Sirona 10 pcs 32.F0019.00 Irrigation set 1/2Y ATR Plus /3000 /5000 10 pcs 32.F0090.00 32.F0106.00 Aspiration System and Suction Tip Yankauer The surgical aspirator with Yankauer suction tip is suitable for all those surgical operations, where an accurate aspiration is needed, so as not to impair the visibility of the operation field. The special nozzles that are placed all around the apical perimeter of the suction tip avoid any accidental suction of small edges of tissue during gingivoplasty. The aspirator is made of medical grade transparent PVC, so as not to absorb the light of shadow-free operating lamps obscuring the surgical site. It is supplied with bending-resistant flexible connectors. Surgical aspirator, 2.50, m with Yankauer suction tip and aspiration control 32.F2044.00 PVC 10 pcs system. (each pack contains 1 adapter Adat. 05) 32.F6361.00 Yankauer sterile suction tip (tip length: 24,5 cm) with aspiration control system PVC 10 pcs 1 Omnia® PTFE Sutures - Black Needle Omnia surgical PTFE sutures are ideal for any implant, periodontal and bone graft surgery where the usage of a monofilament suture with low bacterial adhesion is recommended. Omnia PTFE sutures are soft, biologi- cally inert and chemically non reactive. Compared to other monofilament synthetic sutures, this material is highly tolerated in the oral cavity. -

Chargemaster
Charge Code Description Price Medicare CPT 400481 OR 481 TO 540 MIN 9100 SURG 400777 SUTURES-RUN 92 400778 SUTURE-POP OFF 128 400779 SUTURE-REEL 33 400780 SUTURE-TIES 84 440002 STALEVO 150 11.2 3000817 PNEUM VACCINE ADMINI 57 G0009 3000819 CA SCREEN; PELVIC/BR 103 G0101 3000823 COLORECTAL SCR SCOPE 1030 G0105 3001087 APPLICATION FOREARM 200 29085 3001104 TECH COMP EXC DEB 42 376 11042 3001297 HIPPS 0 MANUL 3001740 CENTRAL VENOUS LINE 1500 36556 3001741 TECH COMP REPLACMENT 1500 36580 3001742 TECH COMP REMOVAL HI 1000 36589 3001745 TECH COMP GASTROSTOM 325 3001746 TECH COMP I&D SCROTA 1700 55100 3001913 PRIVATE ROOM W/BATH 982 3002874 SEMI-PRIVATE 960 3002879 TECH COMP TUBE THORA 900 32551 3002882 TECH COMP REPLACEMEN 1500 36580 3003298 CVP TECHNICAL COMPON 1500 36556 3003882 ADMIN BLD/BLD PRDUCT 565 36430 3004250 TECH COMP INS ART LN 515 36245 3004251 CVP TECHNICAL COMPON 1500 36556 3004253 TECH COMP REPLACEMEN 1500 36580 3004256 TECH COMP BX 45100 3300 45100 3004259 TECH COMP PARACENTES 815 49082 3004261 INSERTION TEMP BLADD 200 51702 3004263 DILATION URETHRA SUB 275 53621 3004264 TECH COMP DILATION U 150 53661 3004538 NURSERY/SICK BABY 450 3004850 TREATMENT ROOM LVL 1 150 99211 3004853 TREATMENT RM LVL 3 250 99213 3004995 TECH COMP PARA REPEA 815 49082 3005022 OBS DIRECT ADMIT 1ST 600 G0379 3005110 SWING BED 441 3005209 OBSERV RM. EACH HR 50 G0378 3005845 INJ INTRA-A THERAPEU 200 96373 3005933 IMMUN ADMIN ONE VACC 53 90471 3005938 IV INF HYDRATION INI 215 96360 3005939 IV INF HYDRATION EAC 65 96361 3005940 IV INF THER PROPH DI 300 96365 -

Corrigendum for Open Surgical Instruments for the Department Of
Date: - 07th September, 2018 Corrigendum For Open Surgical Instruments for the Department of Surgical Oncology NIT Issue Date : 25th July, 2018 NIT No. : Admn/Tender/71/2018-AIIMS.JDH Pre-Bid Meeting : 06th August, 2018 at 04:00 PM Earlier Last Date of Submission : 04th September, 2018 at 03:00 PM Extended Last Date of Submission : 19th September, 2018 at 03:00 PM Bid opening : 20th September, 2018 at 03:15 P.M The following revised and additional specification will be added:- 1. Page No. 11 & 12 For S. No. Name of Surgical Instrument Quantity 1 SS TRAY LARGE 470X320X50MM 4 2 SS TRAY SMALL 350X240X40MM 8 3 KIDNEY DISH LARGE 250X140X40MM 8 4 KIDNEY DISH SMALL 170X100X35MM 10 5 SS BOWL 80X40MM 6 6 SS BOWL 166X50MM 6 7 SSBOWL 160X65MM 8 8 SS BOWL 147X65MM 8 9 SS DRUM LARGE 15X12 INCH 4 10 SS DRUM SMALL 11X9 INCH 4 11 BACKHAUS TOWEL CLAMP 13 CM 64 12 FORSTER SPONGE HOLDER 18 Cm 18 13 BP HANDLE NO. 3 8 14 BP HANDLE NO. 4 7 15 BP HANDLE NO. 7 9 16 SUCTION TIP 2MM 9 17 SUCTION TIP 5MM 8 18 YANKAUER SUCTION TIP 10 MM 4 19 SS SCALE 5 20 DEAVER RETRACTOR SMALL 18CM(TIP 19MM) 14 Corrigendum for Open Surgical Instruments at AIIMS Jodhpur Page 1 21 DEAVER RETRACTOR MEDIUM 30.5CM (TIP 25 MM) 10 22 DEAVER RETRACTOR LARGE 31.5CM (TIP 50MM) 10 23 DOYEN’S RETRACTOR 4 24 MORRIS RETRACTOR 25cm ( BLADE 7x4cm) 6 25 SKIN HOOK 32 26 LANGENBECK RETRACTOR SMALL 16cm (TIP 21x 8mm) 16 27 LANGENBECK RETRACTOR MEDIUM 22cm (TIP 50x11mm) 16 28 LANGENBECK RETRACTOR LARGE 22.5cm (TIP 85x15mm) 14 29 C ZERNY RETRACTOR 17.2 cm 14 30 VEIN RETRACTOR 18 31 BALFOUR ABDOMINAL RETRACTOR 20cm 3 32 MASTOID RETRACTOR 4 33 PERIOSTEUM ELEVATOR SHARP 4 34 PERIOSTEUM ELEVATOR BLUNT 4 35 DISSECTING TOOTH FORCEPS 15 CM 16 36 DISSECTING PLAIN FORCEPS 18 CM 16 37 ARTERY FORCEPS CVD 15 CM 36 38 ARTERY FORCEPS ST. -

Chirurgische Instrumente Surgical Instruments
CHIRURGISCHE INSTRUMENTE SURGICAL INSTRUMENTS SURGICAL INSTRUMENTS Percussion Hammers & Aesthesiometers 01-103 01-102 DEJERINE 01-104 DEJERINE With Needle TAYLOR Size: 200 mm Size: 210 mm Size: 195 mm 01-101 ½ ½ ½ TROEMNER Size: 245 mm ½ 01-109 01-106 01-107 WARTENBERG BUCK RABINER Pinwheal For 01-105 With Needle With Needle 01-108 Neurological BERLINER And Brush And Brush ALY Examination Size: 200 mm Size: 180 mm Size: 255 mm Size: 190 mm Size: 185 mm ½ ½ ½ ½ ½ Page 1 2 Stethoscopes 01-112 01-110 01-111 BOWLES PINARD (Aluminum) aus Holz (Wooden) Stethoscope Size: 155 mm Size: 145 mm With Diaphragm ½ ½ 01-113 01-114 ANESTOPHON FORD-BOWLES Duel Chest Piece 01-115 With Two Outlets BOWLES Page 2 3 Head Mirrors & Head Bands 01-116 01-117 ZIEGLER mm ZIEGLER mm Head mirror only Head mirror only with rubber coating with metal coating 01-118 01-120 ZIEGLER MURPHY Head band of plastic black Head band of celluloid, white 01-119 ZIEGLER Head band of plastic white 01-121 01-122 Head band of plastic, Head mirror with black white, soft pattern plastic head band. Page 3 4 Head Light 01-123 CLAR Head light, 6 volt, with adjustable joint, white celluloid head band, cord with plugs for transformer 01-124 White celluloid head band, only, for 01-125 Spare mirror only, for 01-126 spare bulb 01-127 CLAR Head light, 6 volt, with adjustable joint, white celluloid head band, with foam rubber pad and cord with plugs for transformer 01-128 White celluloid head band, only, for head light 01-129 mirror only, for head light 01-130 spare foam rubber pad, for head band -

Penoscrotal Three-Piece Inflatable Penile Prosthesis Placement: Surgical Technique
Editorial Page 1 of 6 Penoscrotal three-piece inflatable penile prosthesis placement: surgical technique Matthew J. Watson, Anand Shridharani Department of Urology, University of Tennessee-Chattanooga, Chattanooga, TN, USA Correspondence to: Anand Shridharani. Department of Urology, University of Tennessee-Chattanooga, Chattanooga, TN, USA. Email: [email protected]. Received: 06 April 2019; Accepted: 11 November 2019; Published: 05 July 2020. doi: 10.21037/jovs.2019.11.09 View this article at: http://dx.doi.org/10.21037/jovs.2019.11.09 Introduction Table 1 Penoscrotal IPP attributes Advantages of penoscrotal IPP placement The development of the inflatable penile prosthesis (IPP) in 1973 changed the landscape of erectile dysfunction Optimal corporal exposure (ED) management. It is the first and only treatment to Minimal risk of dorsal nerve injury prove efficacious in all males while simulating an erect Optimal approach in severely obese and flaccid state. Subsequent developments in the device, Anchoring of pump directly in scrotum such as new cylinder designs to prevent aneurysm, antibiotic coatings to reduce infection and lockout Optimal approach in patients with severe corporal fibrosis valves to prevent autoinflation, have caused the device Minimal scar to be widely accepted and utilized. Now IPPs more Can perform concomitant scrotoplasy, AUS placement, Mini- closely approximate natural flaccidity, natural erection Jupette (sling) through same incision and are much more reliable. The penoscrotal approach Disadvantages of penoscrotal IPP placement to IPP placement is most common approach utilized Blind placement of reservoir and performed in over 80% of the IPPs placed (1). Although the penoscrotal approach does not allow direct Increased scrotal swelling visualization of the reservoir placement, it likely derives IPP, inflatable penile prosthesis. -

Tyrone Hospital's Charge Master 2019
Tyrone Regional Health Network Charge Master 2019 Disclaimer: *Please note the charges provided are an estimate and is not a guarantee of the final billing charges. The charges are subject to change without notice. Charge Code Description CPT4 HCPCS MA WC Charge 542A0130 AMED WHEELCHAIR VAN - ONE WAY A0426 $214.00 542A0426 AMED EMERGENCY LEVEL 1 ECH WAY A0426 $835.00 542A0428 AMED - BLS A0428 $377.00 542T2005 AMED STRETCHER VAN T2005 $167.00 623A6211 SURGICAL DRESSINGS A6211 $51.00 623A6213 SURGICAL DRESSINGS A6213 $32.00 623A6260 SURGICAL DRESSING A6260 $14.00 623A6441 SURGICAL DRESSING A6441 $6.00 623A6442 SURGICAL DRESSINGS A6442 $3.00 623A6443 SURGICAL DRESSINGS A6443 $4.00 762G0379 DIRECT ADMIT TO OBS G0379 $164.00 11001013 MED/SURG PRIVATE PREM PAT REQ 1013 $15.00 11001020 MED/SURG R & B PRIVATE PAT REQ 1020 $15.00 12001001 MED/SURG R & B SEMI PRIVATE 1001 $749.00 16401012 SWING BED R & B 1012 $642.00 20601005 TELEMETRY R & B 1005 $963.00 27003283 WOUND VAC $126.00 27005165 BINDER XL ABDOMINAL $148.00 27005166 BINDER XXL ABDOMINAL $148.00 27005234 FLANGE 2.75 WAFER A4409 $9.00 27006024 WOUND CLOSURE TRAY $37.00 27008399 KANGAROO EPUMP ENTERAL SET $7.00 27031515 FIRST STEP SELECT $121.00 27055001 MED-SURG SUPPLIES $33.00 27203143 STOMA PASTE A4406 $19.00 27205018 ENDO TUBE 3.5 UNCUFFED MURPHY $4.00 27205102 TRAY SUTURE REMOVAL STERILE $7.00 27205234 FLANGE 2.75 WAFER A4409 $9.00 27205235 POUCH 2.75 OPAGUE A4425 $7.00 27205236 FLANGE 2.25 WAFER A4409 $9.00 27205237 POUCH 2.25 OPAGUE A4425 $7.00 27205238 POUCH 1 PC DURA ADHESIVE A4388 -

General Catalogue 2016/2017
General Catalogue 2016/2017 Omnia, sure to be safe Omnia is a constantly evolving company, aware of the problems that can arise in dental surgeries and ready to create new solutions to meet dentists’ needs. Since 1990, our history is characterized by a growing commitment in the fight against cross-infection, which has enabled us to achieve standards of hygiene and safety that meet the strictest national and international regulations. Our mission: to enable dentists around the world to work in the best possible conditions in terms of hygiene, safety, effectiveness and efficiency. Our numbers: 35 employees, 60 agents in Italy, 3200 square meters of business area, about 400 distributors in Italy and in 50 countries around the world. We celebrated our 25th anniversary and we are now ready to take on the challenges that the future holds for us: • a constant technological development of our products and the ways in which we interact with our customers • the continuous training of dental professionals to help them do their jobs at best • the search for new markets where to introduce Omnia brand and know-how. The customer satisfaction is our major goal and is leading us in all our processes. We are used to face the customer needs in the shortest time, optimizing our knowledge and the individual competences of our team. We favour the know how inside the company sharing the experiences among the collaborators. The high level of expertise and professional skills owned by our team, which Omnia is training internally and externally with the most qualified organisations, allows us to keep our leading position in the dental market. -
JARIT Instruments Are 100% Inspected for Smoothness, Ergonomics and the “Feel” That Surgeons Demand of a Surgical Instrument
CARDIOVASCULAR INSTRUMENTS German Quality • Precision Instruments • Your Confidence C ARDIO V ASCULAR Integra LifeSciences Corporation USA and Canada 311 Enterprise Drive 877-468-5572 Plainsboro, NJ 08536 609-750-4257 (Fax) www.Integra-LS.com JARIT Outside USA and 9 Skyline Drive Canada Hawthorne, NY 10532 914-592-9050 www.jarit.com 914-592-8056 (Fax) 800-431-1123 Printed in USA 10K NS1888-04/08 O U R M I S S I O N I S SINGULARLY DEDICATED TO THE DESIGN, PR O DUCTI O N A N D S E R V I C E O F T H E W O R L D ’ S F I N E S T SURGICAL INSTRUMENTS. O U R S T E A D F A S T C O M M I T M E N T T O T H E S U R G I C A L C O M M U N I T Y PR O V I D E S I N N O VATI O N A N D Q U A L I T Y W I T H I N A U N I Q U E L Y C O S T- E F F E C T I V E DELIVERY SYSTEM. I CardioVascular JARIT... THE INSTRUMENT PEOPLE JARIT IS INSTRUMENTS... For more than 30 years JARIT has provided its customers quality, innovation and integrity in surgical instrumentation. With corporate headquarters in Hawthorne, New York and operation headquarters in Tuttlingen, Germany, JARIT has been able to offer its customers the combination of American ingenuity and German excellence. -

Critical Procedures
chapter 26 Critical Procedures Brent R. King, MMM, MD, FAAP, FACEP, FAAEM Christopher King, MD, FACEP Wendy C. Coates, MD, FACEP Objectives Chapter Outline Demonstrate the Introduction Section 10: Emergent Intubation 1 maneuvers required Section 1: Pediatric Length-Based 10.1 Endotracheal Intubation for the basic treatment Resuscitation Tape 10.2 Rapid Sequence Intubation of ill and injured Section 2: Cervical Spine Section 11: Rescue Airway children, including Stabilization Techniques oxygen administration, Section 3: Monitoring 11.1 Supraglottic Devices monitoring, and basic 3.1 ECG Monitoring 11.1A Laryngeal Mask Airway airway maneuvers. 3.2 Impedance Pneumatography 11.1B Combitube Demonstrate 3.3 Blood Pressure Monitors 11.2 Specialized Laryngoscopes 2 orotracheal intubation 3.4 Pulse Oximetry 11.2A Fiberoptic Scopes using a manikin model. 3.5 Exhaled Carbon Dioxide 11.3 Laryngoscopes With Special Monitors Blades Demonstrate 11.3A Video Laryngoscopes Section 4: Oxygen Administration placement of an 11.3B Lighted Stylet 3 4.1 Nasal Cannula intraosseous needle 11.4 Tracheal Guides 4.2 Oxygen Masks in a manikin or other 4.3 Nonrebreathing Masks Section 12: Surgical Airway model and describe 4.4 Oxygen Hoods Techniques the landmarks for 12.1 Needle Cricothyrotomy Section 5: Suction intraosseous needle 12.2 Retrograde Intubation Section 6: Opening the Airway placement in a child. 12.3 Wire-Guided Cricothyrotomy 6.1 Chin-Lift Maneuver Describe the 12.4 Surgical Cricothyrotomy 6.2 Jaw-Thrust Maneuver indications and 12.5 Tracheostomy Management 4 Section 7: Airway Adjuncts technique for needle Section 13: Cardioversion and 7.1 Oropharyngeal Airways thoracostomy. Defibrillation 7.2 Nasopharyngeal Airways 13.1 Cardioversion and Describe indications Section 8: Bag-Mask Ventilation Defibrillation and technique for 5 Section 9: Management of Upper 13.2 Automated External pericardiocentesis. -

Exam #6: Explanations with References
ASSOCIATION OF SURGICAL TECHNOLOGISTS SURGICAL TECHNOLOGIST CERTIFYING EXAM STUDY GUIDE, 3RD Ed. PRACTICE EXAM #6: EXPLANATIONS WITH REFERENCES Reference Key Alex Rothrock, J. C. (2011). Alexander’s care of the patient in surgery. (14th ed.). St. Louis: Elsevier Anatomy Tortora, G., & Nielsen, M. T. (2009). Principles of human anatomy (11th ed.). Hoboken, NJ: John Wiley & Sons B&K Phillips, N. (2013). Berry & Kohn’s operating room technique (12th ed.). St. Louis: Elsevier. Med Term Gylys, B. A., & Wedding, M. E. (2009). Medical terminology systems: A body systems approach (6th ed.). Philadelphia: F.A. Davis. Memmler’s Cohen, B. J., & Taylor, J. J. (2009). Memmler’s the human body in health and disease (11th ed.). Philadelphia: Lippincott Williams & Wilkins. Micro Price, P., & Frey, K. B. (2003). Microbiology for surgical technologists. Clifton Park, NY: Delmar Learning. OR Instr Tighe, S. M. (2007). Instrumentation for the operating room (7th ed.). St. Louis: Mosby. Pharm Snyder, K. C., & Keegan, C. (2006). Pharmacology for the surgical technologist (2nd ed.). St. Louis: Saunders. Physiology Rhoades, R., & Pflanzer, R. (2003). Human physiology (4th ed.). Pacific Grove, CA: Brooks/Cole. ST for ST Frey, K. B., & Ross, T. (Eds.). (2014 ). Surgical technology for the surgical technologist: A positive care approach (4th ed.). Clifton Park, NY: Delmar Cengage Learning. 1. B. The microvilli, villi, and the plicae circulares increase the surface area for absorption and digestion in the small intestine. (Physiology 713) 2. D. Penfield dissectors are used to separate the dura mater form the cranium during a craniotomy. The Penfield is atraumatic and its use prevents tearing the dura mater. -

Bringing Better Healthcare Products PO BOX 83263- SHARJAH UAE
bringing better healthcare products PO BOX 83263- SHARJAH UAE Tel: +971 6 5501133 | Fax: +971 6 550 1113 Email: [email protected] Web: www.aspenuae.com About Us ASPEN Medical is an emerging healthcare solution provider in United Arab Emirates. We are located in the emirate of Sharjah, and serving the healthcare sector in Dubai, Abu Dhabi, Sharjah and Northern emirates. Our vision is to become the most trustworthy healthcare solution provider in the region through quality and innovative products and technologies. Our mission is to lead the healthcare industry by providing customized, high quality and cost effective solutions in various segments in society, we are dealing with sophisticated high-end medical equipments technologies, consumables. Our well experienced staff with excellent product knowledge provide the best customer service to our customers. We have well qualified and experienced biomedical engineers for carrying our product presentations, installations and after-sale service for our products. Our Major Principals Aspen Surgical USA Iradimed USA Barray USA Medino Gmbh Germany Hirtz & Co Germany Alimed USA Armstrong Medical USA JDH International USA PSC Med USA Viscot LLC USA Busse Inc USA Henniss Germany Morgan Lens USA E Life Orthopedics Taiwan Edan Instruments China Sklar Instruemnts USA MRI Equipments USA Product Range – Medical Equipments General Medical Equipments Patient Monitor / Transport Monitors Vital Signs Monitors , Pulse Oximeters ECG- Holter – ABPM Monitor Defibrillators, AEDs, AED Trainers, Manikins Spirometers