Reflux and Distillation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction of Wax from Sorghum Bran •
i EXTRACTION OF WAX FROM SORGHUM BRAN • 1 HSIEN-WEN HSU <& . ***£/ B. S., National Taiwan University, China, 1951 A THESIS » submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE - Department of Chemical Engineering KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE - 1955 » k Lo i~i ii Q^cu^v^Js TABLE OF CONTENTS INTRODUCTION 1 PREVIOUS WORK 3 MATERIALS 6 GLASS SOXHLET EXTRACTION AND ANALYSIS OF WAX AND OIL IN MISCELLA 7 Procedure of Using Soxhlet Extractor 7 Separation of Wax from Oil by the Acetone Method.. 8 Summary of Results 9 PILOT PLANT EXTRACTION 15 SEPARATION OF WAX AND OIL 16 Effect of Temperature in the Acetone Method 16 Urea Complex Method of Separation. 17 Theory 17 Experimental Procedure 22 Summary of Results 26 COST ESTIMATION OF A WAX RECOVERY PLANT 29 DISCUSSION 30 CONCLUSIONS 35 ACKNOWLEDGMENTS 36 BIBLIOGRAPHY 37 APPENDIX 39 INTRODUCTION i One of the functions of the Agricultural Experiment Station is the never ending search for means of better and fuller utilization of the agri- cultural products that are of particular interest in the state of Kansas. In line with this broad objective, the extraction of economically valuable lipid materials from the sorghum bran has been studied in several theses in the Departments of Chemical Engineering and Chemistry in recent years. Generally the emphasis has b. en placed upon either the chemical and physical properties of these lipides or the equipment design and performance for the solvent ex- traction operation. A brief review of the previous work is presented in the ensuing section. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Distillation 65 Chem 355 Jasperse DISTILLATION
Distillation 65 Chem 355 Jasperse DISTILLATION Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize. The vapors produced are subsequently passed through a water-cooled condenser. Upon cooling, the vapor returns to it’s liquid phase. The liquid can then be collected. The ability to separate mixtures of liquids depends on differences in volatility (the ability to vaporize). For separation to occur, the vapor that is condensed and collected must be more pure than the original liquid mix. Distillation can be used to remove a volatile solvent from a nonvolatile product; to separate a volatile product from nonvolatile impurities; or to separate two or more volatile products that have sufficiently different boiling points. Vaporization and Boiling When a liquid is placed in a closed container, some of the molecules evaporate into any unoccupied space in the container. Evaporation, which occurs at temperatures below the boiling point of a compound, involves the transition from liquid to vapor of only those molecules at the liquid surface. Evaporation continues until an equilibrium is reached between molecules entering and leaving the liquid and vapor states. The pressure exerted by these gaseous molecules on the walls of the container is the equilibrium vapor pressure. The magnitude of this vapor pressure depends on the physical characteristics of the compound and increases as temperature increases. In an open container, equilibrium is never established, the vapor can simply leave, and the liquid eventually disappears. But whether in an open or closed situation, evaporation occurs only from the surface of the liquid. -
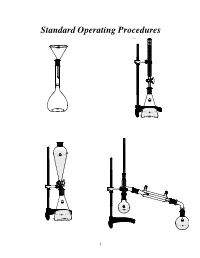
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -

Laboratory Rectifying Stills of Glass 2
» LABORATORY RECTIFYING STILLS OF GLASS 2 By Johannes H. Bruun 3 and Sylvester T. Schicktanz 3 ABSTRACT A complete description is given of a set of all-glass rectifying stills, suitable for distillation at pressures ranging from atmospheric down to about 50 mm. The stills are provided with efficient bubbling-cap columns containing 30 to 60 plates. Adiabatic conditions around the column are maintained by surrounding it with a jacket provided with a series of independent electrical heating units. Suitable means are provided for adjusting or maintaining the reflux ratio at the top of the column. For the purpose of conveniently obtaining an accurate value of the true boiling points of the distillates a continuous boiling-point apparatus is incor- porated in the receiving system. An efficient still of the packed-column type for distillations under pressures less than 50 mm is also described. Methods of operation and efficiency tests are given for the stills. CONTENTS Page I. Introduction 852 II. General features of the still assembly 852 III. Still pot 853 IV. Filling tube and heater for the still pot 853 V. Rectifying column 856 1. General features 856 2. Large-size column 856 3. Medium-size column 858 4. Small-size column 858 VI. Column jacket 861 VII. Reflux regulator 861 1. With variable reflux ratio 861 2. With constant reflux ratio 863 VIII. The continuous boiling-point apparatus 863 IX. Condensers and receiver 867 X. The mounting of the still 867 XI. Manufacture and transportation of the bubbling-cap still 870 XII. Operation of the bubbling-cap still 870 XIII. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -
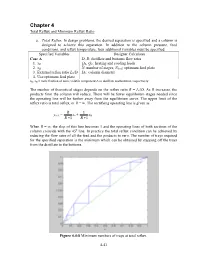
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

Fractional Distillation (Ideal) 1-Propanol/2-Propanol at 760 Torr
Statistical Molecular Thermodynamics Christopher J. Cramer Video 10.7 Azeotropes and Immiscible Phases Fractional Distillation (Ideal) 1-propanol/2-propanol at 760 torr (from Video 10.5) bp as a function of composition x1−propanol(Solution) y1−propanol (Vapor) At 90 °C (between the two pure component boiling points) € the composition of the liquid is 59 mol% 1-propanol (point a) and the vapor is 45 mol% 1-propanol (point b) Fractional distillation: vapor is condensed and revaporized many times a to b to c to d to ... and finally you get to the pure lower boiling component at the top (the coolest point) of a distillation head! Fractional Distillation (Non-Ideal) benzene/ethanol at 760 torr Using Dalton’s/Raoult’s laws: * P2 x2P2 b b y = = a a 2 760 torr lim x2→1 760 torr d c d c and Dalton’s/Henry’s laws: f e e P2 x2kH,2 y2 = = 760 torr lim x2→0 760 torr bp as a function of composition Deviation from ideality permits the vapor phase to be enriched in either component depending on liquid composition. Distillation of benzene-rich mixture Distillation of ethanol-rich mixture Fractional distillation: vapor is condensed and revaporized many times a to b to c to d to e to f, but, irrespective of initial composition, ultimately an azeotropic composition is reached (about 55:45 above) that cannot be further separated by distillation! Self-assessment What purification can be accomplished for a liquid mixture that is not already at the azeotropic composition? Self-assessment Explained If not already at the azeotropic composition, the azeotrope at the top of b b a a the distillation head will be carrying away a greater fraction of one component than d is present in the liquid.