CHEM 344 Distillation of Liquid Mixtures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction of Wax from Sorghum Bran •
i EXTRACTION OF WAX FROM SORGHUM BRAN • 1 HSIEN-WEN HSU <& . ***£/ B. S., National Taiwan University, China, 1951 A THESIS » submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE - Department of Chemical Engineering KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE - 1955 » k Lo i~i ii Q^cu^v^Js TABLE OF CONTENTS INTRODUCTION 1 PREVIOUS WORK 3 MATERIALS 6 GLASS SOXHLET EXTRACTION AND ANALYSIS OF WAX AND OIL IN MISCELLA 7 Procedure of Using Soxhlet Extractor 7 Separation of Wax from Oil by the Acetone Method.. 8 Summary of Results 9 PILOT PLANT EXTRACTION 15 SEPARATION OF WAX AND OIL 16 Effect of Temperature in the Acetone Method 16 Urea Complex Method of Separation. 17 Theory 17 Experimental Procedure 22 Summary of Results 26 COST ESTIMATION OF A WAX RECOVERY PLANT 29 DISCUSSION 30 CONCLUSIONS 35 ACKNOWLEDGMENTS 36 BIBLIOGRAPHY 37 APPENDIX 39 INTRODUCTION i One of the functions of the Agricultural Experiment Station is the never ending search for means of better and fuller utilization of the agri- cultural products that are of particular interest in the state of Kansas. In line with this broad objective, the extraction of economically valuable lipid materials from the sorghum bran has been studied in several theses in the Departments of Chemical Engineering and Chemistry in recent years. Generally the emphasis has b. en placed upon either the chemical and physical properties of these lipides or the equipment design and performance for the solvent ex- traction operation. A brief review of the previous work is presented in the ensuing section. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Single Pass Cooling Systems
A Sustainable Alternative to Single Pass Cooling Systems Amorette Getty, PhD Co-Director, LabRATS What is Single Pass Cooling? ● Used for distillation/reflux condensers, ice maker condensers, autoclaves, cage washers… ● Use a continuous flow of water from faucet to sewer o 0.25-2 gallons per minute, o up to 1,000,000 gallons of water/year if left on continuously Single Pass Cooling: Support ● “U.S. Environmental Protection Agency has ranked the elimination of single-pass cooling systems #4 on its list of top ten water management techniques” -Steve Buratto, Chair of Chemistry and Biochemistry department NOT Just a Sustainability Issue! California Nanosystems Institute (CNSI) flood in a second-floor laboratory in July, 2014. Water ran at ~1-2 gallons per minute for 6-10 hours, overflowing into a ground floor electron microscopy lab CNSI Flood Losses ● $ 2.2 Million in custom built research equipment ● Research shutdown for 4 months ● over 200 hours of staff time ● Insurance no longer covering this type of incident again Campus Responses to the Incident • CNSI ban on single pass cooling systems • TGIF grant financial incentive for optional change of single replacement systems • Chemistry and Material Research Laboratory (MRL) • Additional Grants being sought • Conversation at Campus and UC-level to regulate and replace these types of systems. Seeking Allies… ● “The replacement of single-pass cooling systems with closed-loop and water free systems would save water and represent a major advance in the sustainability efforts of the campus.” -Steve Buratto, Chair of Chemistry and Biochemistry department Barriers to Replacement ● “...major obstacle to replacing the single-pass cooling systems with closed-loop and water- free systems is cost. -

CHEMISTRY ASSIGNMENT CLASS VII CHAP 3, Part – II Elements , Compounds and Mixture ( Separation Techniques of Mixtures )
CHEMISTRY ASSIGNMENT CLASS VII CHAP 3, Part – II Elements , compounds and Mixture ( Separation Techniques of Mixtures ) Mixture can be separated into its constituent by various method. Seperation technique totally depend upon the nature of the constituent. Q1. Describe a method to separate solid to solid mixture . Ans . We can separate solid to solid mixture by using Solvent Method , when one of the component is soluble. In this method we use a appropriate solvent to dissolve one of the component of the mixture .After that we filter the solute .The soluble solute get filtered from the insoluble solid. The filtered solution can be further separated from the solvent by heating or keeping in the sun.In this way we can separate out the two mixture. Q2. Differentiate between Solute and Solvent. Ans Solute Solvent The solid that is dissolved or spread evenly in the The liquid in which solute is dissolved is called solvent is called Solute . solvent. e.g. In sugar syrup sugar is the solute. e.g. In sugar syrup water is the solvent Q3. Name the various method used to separate solid to liquid mixture. Ans. The solid to liquid mixtures can be separated by various method – i. Evaporation ii. Filtration iii. Distillation Q4. What do you mean by filtration ? Draw a well labelled diagram to show filtration. Give one example too. Ans. Filtration is the simplest method to separate mixture when it contain one insoluble solid component and a liquid component by using a filter paper. The clear liquid that passes through the filterpaper is called Filtrate . -

Distillation 65 Chem 355 Jasperse DISTILLATION
Distillation 65 Chem 355 Jasperse DISTILLATION Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize. The vapors produced are subsequently passed through a water-cooled condenser. Upon cooling, the vapor returns to it’s liquid phase. The liquid can then be collected. The ability to separate mixtures of liquids depends on differences in volatility (the ability to vaporize). For separation to occur, the vapor that is condensed and collected must be more pure than the original liquid mix. Distillation can be used to remove a volatile solvent from a nonvolatile product; to separate a volatile product from nonvolatile impurities; or to separate two or more volatile products that have sufficiently different boiling points. Vaporization and Boiling When a liquid is placed in a closed container, some of the molecules evaporate into any unoccupied space in the container. Evaporation, which occurs at temperatures below the boiling point of a compound, involves the transition from liquid to vapor of only those molecules at the liquid surface. Evaporation continues until an equilibrium is reached between molecules entering and leaving the liquid and vapor states. The pressure exerted by these gaseous molecules on the walls of the container is the equilibrium vapor pressure. The magnitude of this vapor pressure depends on the physical characteristics of the compound and increases as temperature increases. In an open container, equilibrium is never established, the vapor can simply leave, and the liquid eventually disappears. But whether in an open or closed situation, evaporation occurs only from the surface of the liquid. -
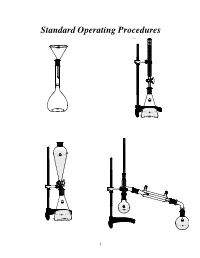
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

Experiment 2 — Distillation and Gas Chromatography
Chem 21 Fall 2009 Experiment 2 — Distillation and Gas Chromatography _____________________________________________________________________________ Pre-lab preparation (1) Read the supplemental material on distillation theory and techniques from Zubrick, The Organic Chem Lab Survival Manual, and the section on Gas Chromatography from Fessenden, Fessenden, and Feist, Organic Laboratory Techniques, then read this handout carefully. (2) In your notebook, write a short paragraph summarizing what you will be doing in this experiment and what you hope to learn about the efficiencies of the distillation techniques. (3) Sketch the apparatus for the simple and fractional distillations. Your set-up will look much like that shown on p 198 of Zubrick, except that yours will have a simple drip tip in place of the more standard vacuum adaptor. (4) Look up the structures and relevant physical data for the two compounds you will be using. What data are relevant? Read the procedure, think about the data analysis, and decide what you need. (5) Since you have the necessary data, calculate the log of the volatility factor (log α) that you will need for the theoretical plate calculation. Distillation has been used since antiquity to separate the components of mixtures. In one form or another, distillation is used in the manufacture of perfumes, flavorings, liquors, and a variety of other organic chemicals. One of its most important modern applications is in refining crude oil to make fuels, lubricants, and other petrochemicals. The first step in the refining process is separation of crude petroleum into various hydrocarbon fractions by distillation through huge fractionating columns, called distillation towers, that are hundreds of feet high. -

22 Bull. Hist. Chem. 8 (1990)
22 Bull. Hist. Chem. 8 (1990) 15 nntn Cn r fr l 4 0 tllOt f th r h npntd nrpt ltd n th brr f th trl St f nnlvn n thr prt nd ntn ntl 1 At 1791 1 frn 7 p 17 AS nntn t h 3 At 179 brr Cpn f hldlph 8Gztt f th Untd Stt Wdnd 3 l 1793 h ntr nnnnt rprdd n W Ml "njn h Cht"Ch 1953 37-77 h pn pr dtd 1 l 179 nd nd b Gr Whntn th frt ptnt d n th Untd Stt S M ntr h rt US tnt" Ar rt Invnt hn 199 6(2 1- 19 ttrfld ttr fnjn h Arn hl- phl St l rntn 1951 pp 7 9 20h drl Gztt 1793 (20 Sptbr Qtd n Ml rfrn 1 1 W Ml rfrn 1 pp 7-75 William D. Williams is Professor of Chemistry at Harding r brt tr University, Searcy, Al? 72143. He collects and studies early American chemistry texts. Wyndham D. Miles. 24 Walker their British cultural heritage. To do this, they turned to the Avenue, Gaithersburg, MD 20877, is winner of the 1971 schools (3). This might explain why the Virginia assembly Dexter Award and is currently in the process of completing the took time in May, 1780 - during a period when their highest second volume of his biographical dictionary. "American priority was the threat of British invasion following the fall of Chemists and Chemical Engineers". Charleston - to charter the establishment of Transylvania Seminary, which would serve as a spearhead of learning in the wilderness (1). -

Laboratory Rectifying Stills of Glass 2
» LABORATORY RECTIFYING STILLS OF GLASS 2 By Johannes H. Bruun 3 and Sylvester T. Schicktanz 3 ABSTRACT A complete description is given of a set of all-glass rectifying stills, suitable for distillation at pressures ranging from atmospheric down to about 50 mm. The stills are provided with efficient bubbling-cap columns containing 30 to 60 plates. Adiabatic conditions around the column are maintained by surrounding it with a jacket provided with a series of independent electrical heating units. Suitable means are provided for adjusting or maintaining the reflux ratio at the top of the column. For the purpose of conveniently obtaining an accurate value of the true boiling points of the distillates a continuous boiling-point apparatus is incor- porated in the receiving system. An efficient still of the packed-column type for distillations under pressures less than 50 mm is also described. Methods of operation and efficiency tests are given for the stills. CONTENTS Page I. Introduction 852 II. General features of the still assembly 852 III. Still pot 853 IV. Filling tube and heater for the still pot 853 V. Rectifying column 856 1. General features 856 2. Large-size column 856 3. Medium-size column 858 4. Small-size column 858 VI. Column jacket 861 VII. Reflux regulator 861 1. With variable reflux ratio 861 2. With constant reflux ratio 863 VIII. The continuous boiling-point apparatus 863 IX. Condensers and receiver 867 X. The mounting of the still 867 XI. Manufacture and transportation of the bubbling-cap still 870 XII. Operation of the bubbling-cap still 870 XIII. -

Heterogeneous Azeotropic Distillation
PROSIMPLUS APPLICATION EXAMPLE HETEROGENEOUS AZEOTROPIC DISTILLATION EXAMPLE PURPOSE This example illustrates a high purity separation process of an azeotropic mixture (ethanol-water) through heterogeneous azeotropic distillation. This process includes distillation columns. Additionally these rigorous multi- stage separation modules are part of a recycling stream, demonstrating the efficiency of ProSimPlus convergence methods. Specifications are set on output streams in order to insure the required purity. This example illustrates the way to set "non-standard" specifications in the multi-stage separation modules. Three phase calculations (vapor-liquid- liquid) are done with the taken into account of possible liquid phase splitting. ACCESS Free-Internet Restricted to ProSim clients Restricted Confidential CORRESPONDING PROSIMPLUS FILE PSPS_EX_EN-Heterogeneous-Azeotropic-Distillation.pmp3 . Reader is reminded that this use case is only an example and should not be used for other purposes. Although this example is based on actual case it may not be considered as typical nor are the data used always the most accurate available. ProSim shall have no responsibility or liability for damages arising out of or related to the use of the results of calculations based on this example. Copyright © 2009 ProSim, Labège, France - All rights reserved www.prosim.net Heterogeneous azeotropic distillation Version: March, 2009 Page: 2 / 12 TABLE OF CONTENTS 1. PROCESS MODELING 3 1.1. Process description 3 1.2. Process flowsheet 5 1.3. Specifications 6 1.4. Components 6 1.5. Thermodynamic model 7 1.6. Operating conditions 7 1.7. "Hints and Tips" 9 2. RESULTS 9 2.1. Comments on results 9 2.2. Mass and energy balances 10 2.3. -

Drinking Water Treatment: Distillation Bruce I
® ® University of Nebraska–Lincoln Extension, Institute of Agriculture and Natural Resources Know how. Know now. G1493 (Revised December 2013) Drinking Water Treatment: Distillation Bruce I. Dvorak, Extension Environmental Engineering Specialist Sharon O. Skipton, Extension Water Quality Educator water as it is boiled in the distiller. Such compounds will not Homeowners are increasingly concerned about be completely removed unless another process is used prior contaminants in their water supply that may affect to condensation. See the section in this NebGuide on treat- health or cause taste, odor, or nuisance problems. Dis- ment principles for further discussion of ways distillers may tillation, one of the oldest methods of water treatment, remove VOCs. is an effective method for reducing many impurities The boiling process during distillation generally inacti- found in water. This NebGuide discusses the process vates microorganisms. However, if the distiller is idle for an and related equipment used for household drinking extended period, bacteria can be reintroduced from the outlet water treatment by distillation. spigot and may recontaminate the water. Water Testing Contaminants Removed from Water by Distillation Regardless of which water treatment system is con- Distillation can remove nearly all impurities from sidered, the water first should be tested to determine what water. Compounds removed include sodium, hardness substances are present. Public water systems routinely test compounds such as calcium and magnesium, other dis- for contaminants. Water utilities are required to publish solved solids (including iron and manganese), fluoride, Consumer Confidence Reports (CCRs), which inform con- and nitrate. Operated properly, it effectively inactivates sumers on the source of the water, contaminants present, microorganisms such as bacteria, viruses, and protozoan potential health effects of those contaminants, and methods cysts (though protozoan cysts are not likely to be found in of treatment used by the utility. -

It's Just a Phase!
Bay Area Scientists in School Presentation Plan Lesson Name It’s just a phase!______________ Presenter(s) Kevin Metcalf, David Ojala, Melanie Drake, Carly Anderson, Hilda Buss, Lin Louie, Chris Jakobson California Standards Connection(s): 3rd Grade – Physical Science 3-PS-Matter has three states which can change when energy is added or removed. Next Generation Science Standards: 2nd Grade – Physical Science 2-PS1-1. Plan and conduct an investigation to describe and classify different kinds of materials by their observable properties. 2-PS1-4. Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot. Science & Engineering Practices Disciplinary Core Ideas Crosscutting Concepts Planning and carrying out PS1.A: Structure and Properties Patterns investigations to answer of Matter ・Patterns in the natural and questions or test solutions to ・Different kinds of matter exist human designed world can be problems in K–2 builds on prior and many of them can be observed. (2-PS1-1) experiences and progresses to either solid or liquid, simple investigations, based on depending on temperature. Cause and Effect fair tests, which provide data to Matter can be described and ・Events have causes that support explanations or design classified by its observable generate observable patterns. solutions. properties. (2-PS1-1) (2-PS1-4) ・Plan and conduct an ・Different properties are suited ・Simple tests can be designed to investigation collaboratively to to different purposes. (2-PS1- gather evidence to support or produce data to serve as the 2), (2-PS1-3) refute student ideas about basis for evidence to answer a ・A great variety of objects can causes.