CHEMISTRY ASSIGNMENT CLASS VII CHAP 3, Part – II Elements , Compounds and Mixture ( Separation Techniques of Mixtures )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experiment 2 — Distillation and Gas Chromatography
Chem 21 Fall 2009 Experiment 2 — Distillation and Gas Chromatography _____________________________________________________________________________ Pre-lab preparation (1) Read the supplemental material on distillation theory and techniques from Zubrick, The Organic Chem Lab Survival Manual, and the section on Gas Chromatography from Fessenden, Fessenden, and Feist, Organic Laboratory Techniques, then read this handout carefully. (2) In your notebook, write a short paragraph summarizing what you will be doing in this experiment and what you hope to learn about the efficiencies of the distillation techniques. (3) Sketch the apparatus for the simple and fractional distillations. Your set-up will look much like that shown on p 198 of Zubrick, except that yours will have a simple drip tip in place of the more standard vacuum adaptor. (4) Look up the structures and relevant physical data for the two compounds you will be using. What data are relevant? Read the procedure, think about the data analysis, and decide what you need. (5) Since you have the necessary data, calculate the log of the volatility factor (log α) that you will need for the theoretical plate calculation. Distillation has been used since antiquity to separate the components of mixtures. In one form or another, distillation is used in the manufacture of perfumes, flavorings, liquors, and a variety of other organic chemicals. One of its most important modern applications is in refining crude oil to make fuels, lubricants, and other petrochemicals. The first step in the refining process is separation of crude petroleum into various hydrocarbon fractions by distillation through huge fractionating columns, called distillation towers, that are hundreds of feet high. -

Heterogeneous Azeotropic Distillation
PROSIMPLUS APPLICATION EXAMPLE HETEROGENEOUS AZEOTROPIC DISTILLATION EXAMPLE PURPOSE This example illustrates a high purity separation process of an azeotropic mixture (ethanol-water) through heterogeneous azeotropic distillation. This process includes distillation columns. Additionally these rigorous multi- stage separation modules are part of a recycling stream, demonstrating the efficiency of ProSimPlus convergence methods. Specifications are set on output streams in order to insure the required purity. This example illustrates the way to set "non-standard" specifications in the multi-stage separation modules. Three phase calculations (vapor-liquid- liquid) are done with the taken into account of possible liquid phase splitting. ACCESS Free-Internet Restricted to ProSim clients Restricted Confidential CORRESPONDING PROSIMPLUS FILE PSPS_EX_EN-Heterogeneous-Azeotropic-Distillation.pmp3 . Reader is reminded that this use case is only an example and should not be used for other purposes. Although this example is based on actual case it may not be considered as typical nor are the data used always the most accurate available. ProSim shall have no responsibility or liability for damages arising out of or related to the use of the results of calculations based on this example. Copyright © 2009 ProSim, Labège, France - All rights reserved www.prosim.net Heterogeneous azeotropic distillation Version: March, 2009 Page: 2 / 12 TABLE OF CONTENTS 1. PROCESS MODELING 3 1.1. Process description 3 1.2. Process flowsheet 5 1.3. Specifications 6 1.4. Components 6 1.5. Thermodynamic model 7 1.6. Operating conditions 7 1.7. "Hints and Tips" 9 2. RESULTS 9 2.1. Comments on results 9 2.2. Mass and energy balances 10 2.3. -

Drinking Water Treatment: Distillation Bruce I
® ® University of Nebraska–Lincoln Extension, Institute of Agriculture and Natural Resources Know how. Know now. G1493 (Revised December 2013) Drinking Water Treatment: Distillation Bruce I. Dvorak, Extension Environmental Engineering Specialist Sharon O. Skipton, Extension Water Quality Educator water as it is boiled in the distiller. Such compounds will not Homeowners are increasingly concerned about be completely removed unless another process is used prior contaminants in their water supply that may affect to condensation. See the section in this NebGuide on treat- health or cause taste, odor, or nuisance problems. Dis- ment principles for further discussion of ways distillers may tillation, one of the oldest methods of water treatment, remove VOCs. is an effective method for reducing many impurities The boiling process during distillation generally inacti- found in water. This NebGuide discusses the process vates microorganisms. However, if the distiller is idle for an and related equipment used for household drinking extended period, bacteria can be reintroduced from the outlet water treatment by distillation. spigot and may recontaminate the water. Water Testing Contaminants Removed from Water by Distillation Regardless of which water treatment system is con- Distillation can remove nearly all impurities from sidered, the water first should be tested to determine what water. Compounds removed include sodium, hardness substances are present. Public water systems routinely test compounds such as calcium and magnesium, other dis- for contaminants. Water utilities are required to publish solved solids (including iron and manganese), fluoride, Consumer Confidence Reports (CCRs), which inform con- and nitrate. Operated properly, it effectively inactivates sumers on the source of the water, contaminants present, microorganisms such as bacteria, viruses, and protozoan potential health effects of those contaminants, and methods cysts (though protozoan cysts are not likely to be found in of treatment used by the utility. -

It's Just a Phase!
Bay Area Scientists in School Presentation Plan Lesson Name It’s just a phase!______________ Presenter(s) Kevin Metcalf, David Ojala, Melanie Drake, Carly Anderson, Hilda Buss, Lin Louie, Chris Jakobson California Standards Connection(s): 3rd Grade – Physical Science 3-PS-Matter has three states which can change when energy is added or removed. Next Generation Science Standards: 2nd Grade – Physical Science 2-PS1-1. Plan and conduct an investigation to describe and classify different kinds of materials by their observable properties. 2-PS1-4. Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot. Science & Engineering Practices Disciplinary Core Ideas Crosscutting Concepts Planning and carrying out PS1.A: Structure and Properties Patterns investigations to answer of Matter ・Patterns in the natural and questions or test solutions to ・Different kinds of matter exist human designed world can be problems in K–2 builds on prior and many of them can be observed. (2-PS1-1) experiences and progresses to either solid or liquid, simple investigations, based on depending on temperature. Cause and Effect fair tests, which provide data to Matter can be described and ・Events have causes that support explanations or design classified by its observable generate observable patterns. solutions. properties. (2-PS1-1) (2-PS1-4) ・Plan and conduct an ・Different properties are suited ・Simple tests can be designed to investigation collaboratively to to different purposes. (2-PS1- gather evidence to support or produce data to serve as the 2), (2-PS1-3) refute student ideas about basis for evidence to answer a ・A great variety of objects can causes. -

Distillation1
Distillation1 Distillation is a commonly used method for purifying liquids and separating mixtures of liquids into their individual components. Familiar examples include the distillation of crude fermentation broths into alcoholic spirits such as gin and vodka, and the fractionation of crude oil into useful products such as gasoline and heating oil. In the organic lab, distillation is used for purifying solvents and liquid reaction products. In analyzing a distillation, how do we know the real composition of each collected component? In this lab, we will introduce gas chromatography (GC), which will tell us how pure each fraction we collected is. After the distillation of your unknown is complete, you will analyze both components via GC. See page 11 and 12 for a light discussion on GC. To understand distillation, first consider what happens upon heating a liquid. At any temperature, some molecules of a liquid possess enough kinetic energy to escape into the vapor phase (evaporation) and some of the molecules in the vapor phase return to the liquid (condensation). An equilibrium is set up, with molecules going back and forth between liquid and vapor. At higher temperatures, more molecules possess enough kinetic energy to escape, which results in a greater number of molecules being present in the vapor phase. If the liquid is placed into a closed container with a pressure gauge attached, one can obtain a quantitative measure of the degree of vaporization. This pressure is defined as the vapor pressure of the compound, which can be measured at different temperatures. Consider heating cyclohexane, a liquid hydrocarbon, and measuring its vapor pressure at different temperatures. -

Simulation of the Open Sky Seawater Distillation
Green and Sustainable Chemistry, 2013, 3, 68-88 http://dx.doi.org/10.4236/gsc.2013.32012 Published Online May 2013 (http://www.scirp.org/journal/gsc) The Best Available Technology of Water/Wastewater Treatment and Seawater Desalination: Simulation of the Open Sky Seawater Distillation Djamel Ghernaout Chemical Engineering Department, Saad Dahlab University of Blida, Blida, Algeria Email: [email protected] Received March 16, 2013; revised April 18, 2013; accepted April 26, 2013 Copyright © 2013 Djamel Ghernaout. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This review suggests the concept of the best available technology of water/wastewater treatment and seawater desalina- tion which is in fact a simulation of the seawater distillation at the open sky: coagulation in salty water aerated basin/ coagulation using seawater as coagulant solution with distillation using stored solar energy followed by waterfall on a natural mountain. This natural, green, and technico-economical technology is composed of three steps: the first one is coagulation which may be achieved: 1) in salty water aerated basin (air stripping, AS; dissolved air flotation, DAF) where the raw water is “diluted” in seawater; or 2) in “conventional” coagulation using seawater as coagulant solution instead of alum/ferric salts. The first option seems to be more natural as it simulates river water dilution in seawater and the second one is more practical for “rapid” water consummation. For colloids and microorganisms’ removal, double- layer compression and charge neutralisation, as main coagulation and disinfection mechanisms, would be involved in the first and second options, respectively. -

CHEM 344 Distillation of Liquid Mixtures
CHEM 344 Distillation of liquid mixtures 1. Distillation basics The vaporization of a liquid and condensation of the resulting vapor is the basis of distillation. Organic liquids containing small amounts (<15%) of impurities or non-volatile substances are easily purified by simple distillation, as are liquid mixtures where the difference in boiling point of the components is >70 oC. Fractional distillation is more useful for separating mixtures of liquids where the boiling points of the components differ by <70 oC (see later). A typical simple distillation setup is shown in Figure 1. It consists of a flask containing the liquid to be distilled, an adapter to hold a thermometer and to connect the flask to a water-cooled condenser, and a flask to hold the condensed liquid (the distillate). Figure 1: Apparatus for a simple distillation. 1.1 The distillation flask The distillation flask is a round-bottom flask. The liquid to be distilled should fill the distillation flask to ~50-60% of its capacity. To promote even heating of the liquid, a boiling chip or a magnetic stir bar is added before heat is applied to the distillation flask. The irregular chips provide sites for bubbles of vapor to form, or alternatively the liquid is agitated with the magnetic stirrer as it is being heated. Never add a boiling chip or a stir bar to a hot liquid! Doing so can cause a seemingly calm liquid to boil suddenly and violently. 1 1.2 The distilling adapter The adapter connects the distillation flask, the condenser, and the thermometer. This type of adapter is often referred to as a distillation head. -

Enrichment of Natural Products Using an Integrated Solvent-Free Process: Molecular Distillation
BK1064-ch62_R2_250706 SYMPOSIUM SERIES NO. 152 # 2006 IChemE ENRICHMENT OF NATURAL PRODUCTS USING AN INTEGRATED SOLVENT-FREE PROCESS: MOLECULAR DISTILLATION Fregolente, L.V.; Moraes, E.B.; Martins, P.F.; Batistella, C.B.; Wolf Maciel, M.R.; Afonso, A.P.; Reis, M.H.M. Chemical Engineering School/Separation Process Development Laboratory (LDPS) State University of Campinas – CP 6066 – CEP 13081-970 – Campinas – SP – Brazil E-mail: [email protected] Molecular distillation is a potential process for separation, purification and/or concentration of natural products, usually constituted by complex and thermally sen- sitive molecules, such as vitamins. Furthermore, this process has advantages over other techniques that use solvents as the separating agent, avoiding problems of toxi- city. This process is characterized by short exposure of the distilled liquid to high temperatures, by high vacuum (around 1024 mmHg) in the distillation space and by a small distance between the evaporator and the condenser (around 2 cm). The pro- ductivity of the process is high, considering the concentration of high added value compounds. In this work, the molecular distillation process was applied to enrich borage oil in gamma-linolenic acid (GLA) and also to recover tocopherols from deo- dorizer distillate of soybean oil (DDSO), since these are components of interest for food and pharmaceutical industries. KEYWORDS: gamma-linolenic acid, vitamin E, molecular distillation, deodorizer distillate of soybean oil, borrage oil INTRODUCTION Molecular distillation shows potential in the separation, purification and/or concentration of natural products, usually constituted by complex and thermally sensitive molecules, such as vitamins and polyunsaturated fatty acids, because it can minimize losses by thermal decomposition. -

Treatment of Landfill Leachate RO Concentrate by VMD
International Conference on Circuits and Systems (CAS 2015) Treatment of Landfill Leachate RO Concentrate by VMD Xingxing Qi a, Chaojie Zhang *b, Ying Zhang c State Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, PR China Abstract—Reverse osmosis (RO) concentrate has been one of the concerned environmental pollution since the RO II. MATERIALS AND METHODS membrane is widely applied. RO concentrate has a dramatic impact on the useful life of landfill, surrounding soil and A. Materials ground water. Thus, the RO concentrate is also urgent to be The characteristics of the RO concentrate from Liming solved. In this research, we firstly explored the characteristics landfill are listed in Table I of vacuum membrane distillation (VMD) by monitoring the membrane flux after controlling for three variables: inflow TABLE I. CHARACTERISTICS OF THE RO CONCENTRATE QUALITY temperature, inflow flux and vacuum degree downstream and found out the optimum operating conditions. Then we further Index Value Index Value tracked the running instances of the system by testing the pH 7.23 Na (mg/L) 3914 membrane flux and the effluent pH, conductivity, TOC and COD at two different temperature. As for the membrane Conductivity(μS/cm) 51400 Mg (mg/L) 498 fouling and wetting, a SEM/EDX analysis was conducted and BOD5 (mg/L) 365 Al (mg/L) 14.50 an efficient solution was given. Finally, we found out that when COD (mg/L) 7450 Zn (mg/L) 0.80 the vacuum pressure is set at 0.08Mpa, inflow flux is set at Cr TOC (mg/L) 1820 Fe (mg/L) 2.89 200L/h and at 75℃ or 80℃, we can get a relatively ideal membrane flux. -

Combining Distillation and Crystallization
© BASF Polyurethane Combining Distillation and Crystallization MANFRED STEPANSKI Methylene diphenyl diisocyanate – in short MDI – PETER FÄSSLER is an important raw material in the manufacture of SULZER CHEMTECH polyurethanes. Sulzer Chemtech has developed a process which, in comparison with conventional processes and through the combination of distil- lation and crystallization, enables MDI to be manu- factured with appreciably lower capital investment and operating costs, as well as with a reduced consumption of energy. Polyurethanes are out-and- Combining Successful out multipurpose plastics. Processes They are used primarily in the MDI results from a reaction of manufacture of rigid and flexible methylene dianiline with phos- foams. Due to their diverse appli- gene. The reaction forms a mix- cation possibilities, polyurethanes ture of monomeric MDI-isomers have a wide range of usage in the (Fig. 2) and components of higher automotive and electrical indus- molecular weight. Monomeric tries, for thermal and cold in- MDI is isolated from this mixture sulation, by the construction of in a number of purification stages. technical components and in the Conventional processes separate furniture industry. Nevertheless, the isomers by means of either dis- polyurethanes are more than just tillation or melt crystallization. foam. They can also be used as Sulzer has developed a hybrid coatings and adhesives, as well as process that combines the two for the manufacture of highly technologies (Fig. 3): After the re- stressed rollers and drums (Fig. 1). action, the crude MDI is distilled 14 SULZER TECHNICAL REVIEW 4/2002 4073 1 The majority of the wheels for in-line skates, microscooters or kickboards are made of polyurethane. -

Azeotropes As Powerful Tool for Waste Minimization in Industry and Chemical Processes
molecules Review Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes Federica Valentini and Luigi Vaccaro * Laboratory of Green S.O.C., Dipartimento di Chimica, Biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy; [email protected] * Correspondence: [email protected]; Tel./Fax: +39-07-5585-5541 Academic Editor: Derek J. McPhee Received: 17 October 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: Aiming for more sustainable chemical production requires an urgent shift towards synthetic approaches designed for waste minimization. In this context the use of azeotropes can be an effective tool for “recycling” and minimizing the large volumes of solvents, especially in aqueous mixtures, used. This review discusses the implementation of different kinds of azeotropic mixtures in relation to the environmental and economic benefits linked to their recovery and re-use. Examples of the use of azeotropes playing a role in the process performance and in the purification steps maximizing yields while minimizing waste. Where possible, the advantages reported have been highlighted by using E-factor calculations. Lastly azeotrope potentiality in waste valorization to afford value-added materials is given. Keywords: azeotrope; waste minimization; solvent recovery; azeotropic distillation; process design; waste valorization 1. Introduction The materials not incorporated into the final desired product and the energy required for conducting a process can be considered most trivially as the major direct origin of “waste” associated to chemical production. In addition, the possible formation of undesired isomers or byproducts also results in the generation of costly waste, that being in some cases hazardous, can limit the final application of the main product. -
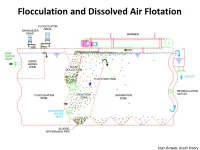
Flocculation and Dissolved Air Flotation
Flocculation and Dissolved Air Flotation Sean Bowen, Aleah Henry Cost Estimation Biomass production/yr 25 tonnes Tank Surface Area 940.5 m2 Yearly operation period 330 days (18h/d) Energy costs $7692 per year Flocculant $15000/year Capital interest for investment 16.5%/yr Unit Description Includes: tankage, pumps, motors, internal piping, c/s package unit, building, air compressor, mix tank, control panel Total BM Cost 2011 (assume error $4,722,400 +-20%) [1] US Department of Energy. National Algal Biofuels Technology Roadmap. Energy Efficiency and Renewable Energy. May 2010. Pages 37-101 [2] Chun-Yen Chen, Kuei-Ling Yeh, Rifka Aisyah, Duu-Jong Lee, Jo-Shu Chang, Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review, Bioresource Technology, Volume 102, Issue 1, January 2011, Pages 71-81 Rotary Vacuum Drum Washers In Kraft Mill Pulping • Each washer operates at 80% filtering efficiency therefore 3 washer in series are connected • In separation of pulp it is the cake formation • Approximately 7 tons of black liquor form for each ton of pulp Design Criteria • Minimum cake thickness is 6 mm • Effective filtration rate per unit area of the drum surface area depends on Figure 2: Rotary vacuum drum submerged circumference filter • The drum takes up 푚 푝푢푙푝 approximately 15% by 퐴푑푟푢푚 = 푚 푓푖푙푡,푓푖푛 volume of the entire washer unit • Rotation speed depends on the time spent in each zone Vacuum Drum Washer Costs Equipment Costs Wash water costs Ultrafiltration • based on the ability of pressure-driven filtration membranes to separate multicomponent solutes according to molecular size, shape, and chemical bonding • Hydraulic pressure drives substances with a smaller molecular size through a membrane while the larger molecules are held back Annual Operating Costs • Capital Cost for Ultrafiltration Membrane Separation unit: $31,324 to $73,088 one time fee.