Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Embryology and Anatomy of Fetal Heart
Prof. Saeed Abuel Makarem Dr. Jamila El Medany Objectives • By the end of this lecture the student should be able to: • Describe the formation, sit, union divisions of the of the heart tubes. • Describe the formation and fate of the sinus venosus. • Describe the partitioning of the common atrium and common ventricle. • Describe the partitioning of the truncus arteriosus. • List the most common cardiac anomalies. • The CVS is the first major system to function in the embryo. • The heart begins to beat at (22nd – 23rd ) days. • Blood flow begins during the beginning of the fourth week and can be visualized by Ultrasound Doppler Notochord: stimulates neural tube formation Somatic mesoderm Splanchnic mesoderm FORMATION OF THE HEART TUBE • The heart is the first functional organ to develop. • It develops from Splanchnic Mesoderm in the wall of the yolk sac (Cardiogenic Area): Cranial to the developing Mouth & Nervous system and Ventral to the developing Pericardial sac. • The heart primordium is first evident at day 18 (as an Angioplastic cords which soon canalize to form the 2 heart tubes). • As the Head Fold completed, the developing heart tubes change their position and become in the Ventral aspect of the embryo, Dorsal to the developing Pericardial sac. • . Development of the Heart tube • After Lateral Folding of the embryo, the 2 heart tubes approach each other and fuse to form a single Endocardial Heart tube within the pericardial sac. • Fusion of the two tubes occurs in a Craniocaudal direction. What is the • The heart tube grows faster than shape of the the pericardial sac, so it shows 5 alternate dilations separated by Heart Tube? constrictions. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Original Articles
Artigo Original %DVHV0RUIROyJLFDVSDUDR(VWXGRGR6HSWR,QWHUDWULDOQR)HWR Humano Morphological Basis for the Study of the Interatrial Septum in the Human Fetus Hugo Becker Amaral, Paulo Zielinsky, Aron Ferreira da Silveira, Ijoni Costabeber, Luiz Henrique Nicoloso, Olmiro Cezimbra de Souza Filho, Marcelo Salum, João Luiz Manica, Juliana Silveira Zanettini, Ane Micheli Costabeber 8QLGDGHGH&DUGLRORJLD)HWDOGR,QVWLWXWRGH&DUGLRORJLDGR5LR*UDQGHGR6XO'HSDUWDPHQWRGH0RUIRORJLDGR&HQWURGH&LrQFLDGD6D~GHGD Universidade Federal de Santa Maria – Porto Alegre, RS Resumo Objetivo: Descrever observações morfológicas sobre o septo interatrial em fetos normais, especialmente o forame oval e o septo primeiro, de forma a comparar a excursão do septo primeiro com o diâmetro do forame oval. Métodos: As medidas da excursão do septo primeiro (ESP) em direção ao átrio esquerdo (AE) e do diâmetro do forame oval (DFO) foram realizadas em corações de dez fetos humanos formolizados com 28 a 36 semanas. Os cortes histológicos foram feitos no FO, SP, septo segundo e nos AE e AD. Resultados: Os resultados da análise anatômica estão expressos em amplitude das medidas do DFO e da ESP: 3 fetos com idade gestacional (IG) presumida de 28 semanas, DFO (3,1-3,5 mm) e ESP (2,8-3,1 mm); 4 fetos com IG presumida de 34 semanas, DFO (3,3-3,5 mm) e ESP (4,0-5,0 mm); e 3 fetos com IG presumida de 36 semanas, DFO (3,3-4,5 mm) e ESP (6,0-9,0). Foram identificadas fibras musculares cardíacas no SP e no segundo. Conclusão: Pode-se sugerir que o SP apresenta caráter ativo devido às fibras musculares que o constituem, influenciando o fluxo sangüíneo através do FO, a mobilidade do SP e a sua excursão para o interior do AE. -

Myocardial Velocities, Dynamics of the Septum Primum, and Placental Dysfunction in Fetuses with Growth Restriction
138 Myocardial Velocities, Dynamics of the Septum Primum, and Placental Dysfunction in Fetuses with Growth Restriction Alexandre Antonio Naujorks, MD, PhD, Paulo Zielinsky, MD, PhD, Caroline Klein, MD, Luiz Henrique Nicoloso, MD, PhD, Antonio Luis Piccoli Jr, MD, PhD, Eduardo Becker, MD, PhD, Renato Frajndlich, MD, PhD, Patricia Pizzato, MD, Carolina Barbisan, MD, Stefano Busato, MD, and Mauro Lopes, MD Fetal Cardiology Unit, Instituto de Cardiologia/Fundação Universitária de Cardiologia, Porto Alegre, Rio Grande do Sul, Brazil ABSTRACT Introduction. Diastolic dysfunction may occur in fetuses with intrauterine growth restriction (IUGR) and may be assessed by myocardial tissue Doppler (MTD). We previously have shown that excursion index of the septum primum (EISP) is reduced in IUGR fetuses over 30 weeks because of a higher left atrial pressure. Patients, Setting, and Design. The sample was made up of 14 fetuses with IUGR. MTD examination was carried out with the sample volume placed at the basal lateral wall of the left ventricle (LV), interventricular septum (IVS), and free wall of the right ventricle (RV) to determine E′/A′ ratios. EISP was calculated as the ratio between the maximal excursion of the septum primum into the left atrium during diastole and the maximal diastolic diameter of the left atrium. Mitral and tricuspid flows were assessed by the conventional Doppler method. Outcome Measures. Pearson’s correlation test was used to analyze the correlations between the parameters. Results. A positive correlation was observed between UARI and E′/A′ ratios for RV (r = 0.63, P = .02), IVS (r = 0.59, P = .03), and LV (r = 0.41, P = .15). -

Development of Heart Notes
Formation of heart tube: 3rd week Heart beat: 22nd –23rd day (beginning of fourth week) USG detection of heart beat: 7th week Foetal ECG: 11th week Endocardium from original heart tube Myocardium from surrounding mesoderm & epicardium (myoepicardial mantle) (visceral pericardium) Lining of pericardium epithelium of pericardial cavity Transverse sinus formed by disappearance of dorsal mesocardium (Present between arterial and venous ends of the heart tube) FATE Of SINUS VENOSUS Left horn of sinus venosus, along with medial part of common cardinal vein forms coronary sinus Lateral part of common cardinal vein forms oblique vein of left atrium Left venous valve merges with septum secundum. Right venous valve is divided in three parts by appearance of two transverse muscular bands, called limbic bands. i) The part above superior limbic band forms crista terminalis ii) The part between the two bands forms valve of inferior vena cava iii) The part below the inferior limbic band forms valve of coronary sinus INTERATRIAL SEPTUM i) Upper, thicker part is formed by septum secundum ii) Lower, thin part (floor of fossa ovalis) is formed by septum primum iii) Sharp margin of fossa ovalis is formed by lower, curved margin of septum secundum DEVELOPMENT OF RIGHT ATRIUM It develops from 1. Right half of primitive atrial chamber (rough part); 2. Absorption of right horn of sinus venosus (smooth part) and 3. Right atrioventricular canal. DEVELOPMENT OF LEFT ATRIUM It develops from 1. Left half of primitive atrial chamber (rough part – confined to the auricle); 2. Absorption of pulmonary veins (smooth part) and 3. Left atrioventricular canal. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography -

The Human Embryonic Heart in the Ninth Week
THE HUMAN EMBRYONIC HEART IN THE NINTH WEEK RICHARD H. LICATA Department of Aqiatoniy, Univeisily of Michigan Medical School, Ann ArboT SIXTEEN I'IQURES Although observations on the developing heart go back to the time of Aristotle, it was not until late in the 19th century that the work of His (1885, 1886) and Born (1888, 1889) resulted in an understanding of heart development that was of sdlicient accuracy to be still of basic value by present standards. One of the crucial factors in their work was the development of adequate methods of reconstruction. Although His was the first to attempt sucli reconstructions, his efforts met with limited success and he had to resort to freehand modelling of embryonic hearts. These models, reproduced commercially by Ziegler, are fairly accurate, though they lack the detail of models made by the wax-plate method. In 1883 Born began to employ the wax-plate reconstruction method as a means of studying heart development. This technique was improved by Kastschenko (1886) and Strasser (1887). Later, the continued efforts of Born and Strasser brought this technique to a high degree of accuracy. A description of their methods was published in detail by Born in 1889. Tandler ('12, '13) greatly furthered knowledge of heart development by coordinating the results of earlier workers and supplementing them with extensive contributions of his own. Mall's work ('12) on the structure of the ventricles, and his observations on the development of the conduction sys- This paper represents x condensation of one section of a dissertation siili- rnitted in partial fulfillment of the requirements for the dqree of Doctor of Philosophy in the University of Michigan. -

Successful Percutaneous Closure of Spiral Atrial Septal Defect
M Alobaidan and others Successful percutaneous closure ID: 14-0101; March 2015 of spiral atrial septal defect DOI: 10.1530/ERP-14-0101 Open Access CASE REPORT Successful percutaneous closure of spiral atrial septal defect Mashail Alobaidan, A Saleem, H Abdo and J Simpson1 Correspondence Department of Pediatric Cardiology, Armed Forces Hospital, Abubaker Alrazi Street, Sulaimania District, should be addressed Riyadh 11199, Saudi Arabia to J Simpson 1Department of Congenital Heart Disease, Evelina London Children’s Hospital, Westminster Bridge Road, Email London, UK [email protected] Summary The case report of a 15-year-old patient with an unusual form of atrial septal defect is described. Echocardiography showed separation of the secundum and primum atrial septums due to abnormal posterior and leftward attachment of the primum septum into the roof of the left atrium. The morphology has been variably described as a ‘double’ atrial septum or ‘spiral’ atrial septal defect. Despite the technical challenge of this form of atrial septal defect, it was effectively closed by ensuring that all relevant septal structures were incorporated between the discs of the occlusion device. This was associated with a stable position and good medium-term outcome. This contrasts with the experience of others where device embolisation or technical failure has been described. Learning points: † The spiral atrial septal defect is characterised by an apparently ‘double’ atrial septum. † Such atrial septal defects (ASDs) have been associated with a high rate of technical failure of transcatheter closure. † 3D echocardiography assists in understanding the anatomy of the defect. † Following deployment of the ASD occlusion device transoesophageal echocardiography is essential to ensure that both septum primum and secundum are between the occluder discs. -

A Critical Role for the Epha3 Receptor Tyrosine Kinase in Heart Development ⁎ Lesley J
Developmental Biology 302 (2007) 66–79 www.elsevier.com/locate/ydbio A critical role for the EphA3 receptor tyrosine kinase in heart development ⁎ Lesley J. Stephen a, Amy L. Fawkes a, Adam Verhoeve a, Greg Lemke b, Arthur Brown a, a BioTherapeutics Research Group, The John P. Robarts Research Institute, and The University of Western Ontario, London, Ontario, Canada N6A 5K8 b Molecular Neurobiology Laboratory, The Salk Institute for Biological Studies, La Jolla, CA 92037, USA Received for publication 29 May 2006; revised 23 August 2006; accepted 24 August 2006 Available online 30 August 2006 Abstract Eph proteins are receptor tyrosine kinases that control changes in cell shape and migration during development. We now describe a critical role for EphA3 receptor signaling in heart development as revealed by the phenotype of EphA3 null mice. During heart development mesenchymal outgrowths, the atrioventricular endocardial cushions, form in the atrioventricular canal. This morphogenetic event requires endocardial cushion cells to undergo an epithelial to mesenchymal transformation (EMT), and results in the formation of the atrioventricular valves and membranous portions of the atrial and ventricular septa. We show that EphA3 knockouts have significant defects in the development of their atrial septa and atrioventricular endocardial cushions, and that these cardiac abnormalities lead to the death of approximately 75% of homozygous EphA3−/− mutants. We demonstrate that EphA3 and its ligand, ephrin-A1, are expressed in adjacent cells in the developing endocardial cushions. We further demonstrate that EphA3−/− atrioventricular endocardial cushions are hypoplastic compared to wildtype and that EphA3−/− endocardial cushion explants give rise to fewer migrating mesenchymal cells than wildtype explants. -
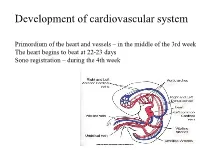
Development of Cardiovascular System
Development of cardiovascular system Primordium of the heart and vessels – in the middle of the 3rd week The heart begins to beat at 22-23 days Sono registration – during the 4th week • Primitive blood circulation. • Heart development (dev. of heart tube, septa and valves) • Aortal arches and their derivatives. • Fetal blood circulation. • Cardiovascular system malformations. Vessels development: (from week 3) hemangiogenesis - blood islands (insulae sanguinae) DAY 15 – 16 in extraembryonic mesoderm of - yolk sac (vasa omphalomesenterica /vitellina/), - connecting stalk and placenta (vasa umbilicalia) DAY 17 – 18 in mesenchyme of embryo Groups of angiogenic cells in mesenchyma ectoderm Blood islet mesenchyme angioblasts endoderm hemoblasts primitive erythrocytes primitive endothelium Differentiation of mesenchymal cells angiogenic cells: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion with tubes forms the cardiogenic area two heart tubes, while the lateral portions form the dorsal aortae. Cardiogenic region just cranial to the prechordal plate. Fusion of heart tubes mesocardium dorsale diferentiation of heart wall: endocardium ● heart jelly epimyocardium Vitelline, umbilical and intraembryonic vessels fuse together and form the primitive blood circulation ( fetal blood circulation!) Pericardial cavity Cor tubulare simplex Septum transversum branchial arteries 1-6 Right Leftt Desc.aorta Ventral wiew Truncus arteriosus Asc.aorta Sinus venosus right horn degenerate Saccus aorticus Vessels v. cardinalis ant. sin. + v. cardinalis com.sin. cor tubulare Yolk sac v. cardinalis post. sin. aa.omphalomesentericae a. mesenterica sin.