Chapter 1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Subalpine Larch (Larix Lyallii), Western Larch (Larix Occidentalis), and Tamarack (Larix Laricina)
Unclassified ENV/JM/MONO(2007)7 Organisation de Coopération et de Développement Economiques Organisation for Economic Co-operation and Development 16-May-2007 ___________________________________________________________________________________________ English - Or. English ENVIRONMENT DIRECTORATE JOINT MEETING OF THE CHEMICALS COMMITTEE AND Unclassified ENV/JM/MONO(2007)7 THE WORKING PARTY ON CHEMICALS, PESTICIDES AND BIOTECHNOLOGY Series on Harmonisation of Regulatory Oversight in Biotechnology No. 41 CONSENSUS DOCUMENT ON THE BIOLOGY OF THE NATIVE NORTH AMERICAN LARCHES: SUBALPINE LARCH (Larix lyalli), WESTERN LARCH (Larix occidentalis) AND TAMARACK (Larix laricina) English - Or. English JT03227278 Document complet disponible sur OLIS dans son format d'origine Complete document available on OLIS in its original format ENV/JM/MONO(2007)7 Also published in the Series on Harmonisation of Regulatory Oversight in Biotechnology: No. 1, Commercialisation of Agricultural Products Derived through Modern Biotechnology: Survey Results (1995) No. 2, Analysis of Information Elements Used in the Assessment of Certain Products of Modern Biotechnology (1995) No. 3, Report of the OECD Workshop on the Commercialisation of Agricultural Products Derived through Modern Biotechnology (1995) No. 4, Industrial Products of Modern Biotechnology Intended for Release to the Environment: The Proceedings of the Fribourg Workshop (1996) No. 5, Consensus Document on General Information concerning the Biosafety of Crop Plants Made Virus Resistant through Coat Protein Gene-Mediated Protection (1996) No. 6, Consensus Document on Information Used in the Assessment of Environmental Applications Involving Pseudomonas (1997) No. 7, Consensus Document on the Biology of Brassica napus L. (Oilseed Rape) (1997) No. 8, Consensus Document on the Biology of Solanum tuberosum subsp. tuberosum (Potato) (1997) No. 9, Consensus Document on the Biology of Triticum aestivum (Bread Wheat) (1999) No. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Phylogeny and Phylogenetic Nomenclature of the Campanulidae Based on an Expanded Sample of Genes and Taxa
Systematic Botany (2010), 35(2): pp. 425–441 © Copyright 2010 by the American Society of Plant Taxonomists Phylogeny and Phylogenetic Nomenclature of the Campanulidae based on an Expanded Sample of Genes and Taxa David C. Tank 1,2,3 and Michael J. Donoghue 1 1 Peabody Museum of Natural History & Department of Ecology & Evolutionary Biology, Yale University, P. O. Box 208106, New Haven, Connecticut 06520 U. S. A. 2 Department of Forest Resources & Stillinger Herbarium, College of Natural Resources, University of Idaho, P. O. Box 441133, Moscow, Idaho 83844-1133 U. S. A. 3 Author for correspondence ( [email protected] ) Communicating Editor: Javier Francisco-Ortega Abstract— Previous attempts to resolve relationships among the primary lineages of Campanulidae (e.g. Apiales, Asterales, Dipsacales) have mostly been unconvincing, and the placement of a number of smaller groups (e.g. Bruniaceae, Columelliaceae, Escalloniaceae) remains uncertain. Here we build on a recent analysis of an incomplete data set that was assembled from the literature for a set of 50 campanulid taxa. To this data set we first added newly generated DNA sequence data for the same set of genes and taxa. Second, we sequenced three additional cpDNA coding regions (ca. 8,000 bp) for the same set of 50 campanulid taxa. Finally, we assembled the most comprehensive sample of cam- panulid diversity to date, including ca. 17,000 bp of cpDNA for 122 campanulid taxa and five outgroups. Simply filling in missing data in the 50-taxon data set (rendering it 94% complete) resulted in a topology that was similar to earlier studies, but with little additional resolution or confidence. -

Vascular Plants of the Sapa Bog Joanne Kline University of Wisconsin - Milwaukee
University of Wisconsin Milwaukee UWM Digital Commons Field Station Bulletins UWM Field Station Spring 1991 Vascular plants of the Sapa Bog Joanne Kline University of Wisconsin - Milwaukee Follow this and additional works at: https://dc.uwm.edu/fieldstation_bulletins Part of the Forest Biology Commons, and the Zoology Commons Recommended Citation Kline, J. 1991. Vascular plants of the Sapa Bog. Field Station Bulletin 24(1): 1-13. This Article is brought to you for free and open access by UWM Digital Commons. It has been accepted for inclusion in Field Station Bulletins by an authorized administrator of UWM Digital Commons. For more information, please contact [email protected]. Vascular Plants of the Sapa Bog Joanne Kline University of Wisconsin-Milwaukee Field Station 3095 Blue Goose Road, Saukville, Wisconsin 53080 Abstract The vascular plants occurring within an acidic black spruce bog and its surrounding moat in southeastern Wisconsin are listed with observational notes on abundance and habitat. Of the 156 species, at least 20 are at or near the southern extent of their range, and four are currently State designated species of Special Concern. Introduction A bog community results from an interaction of climate and hydrogeology. The relative rates of precipitation and evaporation, temperature, bedrock geology and the length of time water is in contact with mineral soil all influence the formation and the type of peat- filled wetland which develops. In general, two types of peatlands occur in glaciated portions of Wisconsin: low nutrient or "ombrotrophic" bogs over granite bedrock, as found in northern Wisconsin, and high nutrient or "minerotrophic" fens over glacial drift and dolomitic bedrock, as found in southeastern Wisconsin. -

A Survey of Tricolpate (Eudicot) Phylogenetic Relationships1
American Journal of Botany 91(10): 1627±1644. 2004. A SURVEY OF TRICOLPATE (EUDICOT) PHYLOGENETIC RELATIONSHIPS1 WALTER S. JUDD2,4 AND RICHARD G. OLMSTEAD3 2Department of Botany, University of Florida, Gainesville, Florida 32611 USA; and 3Department of Biology, University of Washington, Seattle, Washington 98195 USA The phylogenetic structure of the tricolpate clade (or eudicots) is presented through a survey of their major subclades, each of which is brie¯y characterized. The tricolpate clade was ®rst recognized in 1989 and has received extensive phylogenetic study. Its major subclades, recognized at ordinal and familial ranks, are now apparent. Ordinal and many other suprafamilial clades are brie¯y diag- nosed, i.e., the putative phenotypic synapomorphies for each major clade of tricolpates are listed, and the support for the monophyly of each clade is assessed, mainly through citation of the pertinent molecular phylogenetic literature. The classi®cation of the Angiosperm Phylogeny Group (APG II) expresses the current state of our knowledge of phylogenetic relationships among tricolpates, and many of the major tricolpate clades can be diagnosed morphologically. Key words: angiosperms; eudicots; tricolpates. Angiosperms traditionally have been divided into two pri- 1992a; Chase et al., 1993; Doyle et al., 1994; Soltis et al., mary groups based on the presence of a single cotyledon 1997, 2000, 2003; KaÈllersjoÈ et al., 1998; Nandi et al., 1998; (monocotyledons, monocots) or two cotyledons (dicotyledons, Hoot et al., 1999; Savolainen et al., 2000a, b; Hilu et al., 2003; dicots). A series of additional diagnostic traits made this di- Zanis et al., 2003; Kim et al., 2004). This clade was ®rst called vision useful and has accounted for the long recognition of the tricolpates (Donoghue and Doyle, 1989), but the name these groups in ¯owering plant classi®cations. -

Additions to the Boreal Flora of the Northwest Territories with a Preliminary Vascular Flora of Scotty Creek
Additions to the Boreal Flora of the Northwest Territories with a Preliminary Vascular Flora of Scotty Creek MARIE -È VE GARON -L ABRECQUE 1, 2, 6 , É TIENNE LÉVEILLÉ -B OURRET 3, 4 , K ELLINA HIGGINS 5, and OLIVER SONNENTAG 5 1Département des sciences biologiques, Pavillon Marie-Victorin, Université de Montréal, 90 avenue Vincent-d’Indy, Montréal, Québec H3C 3J7 Canada 2Department of Biology, 209 Nesbitt Biology Building, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6 Canada 3Department of Biology, University of Ottawa, 30 Marie Curie, Ottawa, Ontario K1N 6N5 Canada 4Musée canadien de la nature, 1750 chemin Pink, Gatineau, Québec J9J 3N7 Canada 5Département de géographie, Université de Montréal, 520 chemin Côte-Sainte-Catherine, Montréal, Québec H3C 3J7 Canada 6Corresponding author: [email protected] Garon-Labrecque, Marie-Ève, Étienne Léveillé-Bourret, Kellina Higgins, and Oliver Sonnentag. 2015. Additions to the boreal flora of the Northwest Territories with a preliminary vascular flora of Scotty Creek. Canadian Field-Naturalist 129(4): 349–367. We present the first survey of the vascular flora of Scotty Cr eek, a peatland-dominated watershed with discontinuous permafrost about 60 km south of Fort Simpson, Northwest Territories (NWT). Of the 140 vascular plant taxa found at Scotty Creek, two are additions to the boreal flora of NWT: Arethusa bulbosa (Dragon’s-mouth, Orchidaceae) and Carex pauciflora (Few-flowered Sedge, Cyperaceae). The occurrence of Arethusa bulbosa extends the known range of this species 724 km to the northwest, making this purportedly eastern American plant almost pan-Canadian. Two other major range extensions (> 200 km) are reported for Carex brunnescens subsp. -

Subalpine Larch (Larix Lyallii), Western Larch (L. Occidentalis), and Tamarack (L
SECTION 3. NATIVE NORTH AMERICAN LARCHES - 91 Section 3. Native north american larches: subalpine larch (Larix lyallii), western larch (L. occidentalis), and tamarack (L. laricina) Preamble: Each of the three North American larch species is usually discussed separately in each section and subsection of this Consensus Document in the following order: subalpine larch (Larix lyallii), western larch (Larix occidentalis), and tamarack (Larix laricina). 1. Taxonomy Larch forests essentially encircle the colder temperate Northern Hemisphere. Within this area, the larch genus (Larix) is represented by 10-15 species and some subspecies or varieties as well as natural hybrids (Schmidt, 1995; Semerikov and Lascoux 2003; Semerikov et al, 2003). Ten species usually recognised are the North Eurasian Larix decidua, L. sibirica (synonym L. russica), L. gmelinii (including L. cajanderi, L. dahurica, and possibly L. olgensis), and L. kaempferi (synonym L. leptolepis); the South Asian L. griffithiana, L. mastersiana, and L. potaninii; and the North American L. laricina, L. lyallii, and L. occidentalis. All true larches are in the genus Larix Mill., a deciduous needle-leaf lineage in the gymnosperm family Pinaceae. Larch taxonomy has had limited overall attention. This is reflected in a lack of consensus about what constitutes a larch species or subspecies (or botanical variety), and about the phylogenetic relationships among species (Semerikov et al, 2003). The proposed division of Larix into two sections based largely on cone morphology (Vidakovic, 1991) is not supported by studies of chloroplast DNA variation (Qian et al, 1995), nuclear internal transcribed spacer (ITS) region (Gernandt and Liston, 1999), amplified fragment length polymorphisms (AFLPs) (Semerikov et al, 2003), or allozyme variation (Semerikov and Lascoux, 1999; Semerikov et al, 1999). -

Menyanthaceae Buckbean Family
Menyanthaceae buckbean family These widespread species are associated with aquatic habitats. Their leaves are alternate and simple, or Page | 673 sometimes growing in threes. Flowers are perfect although sometimes functionally unisexual on dioecious plants. Corolla is sympetalous, with five lobes. Stamens are attached to the corolla, alternating with the lobes. Fruits may be capsules or berries. Key to genera Leaves with 3 leaflets; emergent; flowers borne in a raceme. Menyanthes Leaves simple, floating; flowers arranged in an umbel. Nymphoides Menyanthes L. Buckbean This is a monotypic genus, circumboreal in range. Leaflets are borne in threes atop a long petiole, sheathing at the base. Flowers are carried on a scape loosely arranged in a raceme, subtended by bracts. Calyx is deeply cleft, its lobes are hirsuteThe corolla is distylic. Menyanthes trifoliata L. Buckbean; trèfle d’eau Leaves are of a leathery texture. Whitish flowers ascend in an erect raceme, scape arising from the base of the plant. Corolla is pubescent within. Flowers in June. Emergent from stagnant pools and bogs. Often dominant. Common at Truro, Kentville, Amherst, to northern Cape Photo by David Mazerolle 3-57 Menyanthaceae Breton. Less frequent southward and known from Sable Island. NL to AK, south to CA, NM, and NC. Page | 674 Photo by Sean Blaney Nymphoides Seguier floating heart Truly aquatic, there are 20 species of these herbs worldwide. Our single native species is dioecious. Leaves either arise directly from a rhizome on long floating petioles, or on petioles arising at the base of the inflorescence. Calyx is lobed, the five segments oblong. The corolla is also cleft into five pubescent lobes. -
Vascular Plant List Deception Pass Deception Pass, Island and Skagit Counties, WA
Vascular Plant List Deception Pass Deception Pass, Island and Skagit Counties, WA. List covers plants found in Deception Pass State Park, including areas on both sides of Deception Pass. WNPS list over several years, through 2000. 238 spp. * - Introduced Scientific Name Common Name Family Name Family Common Color Ref Acer Glabrum var.douglasii Douglas Maple Aceraceae Maple Family White Acer macrophyllum Big-leaf maple Aceraceae White Daucus pusillus American carrot Apiaceae Parsley Family White Glehnia leiocarpa American glehnia Apiaceae White Heracleum lanatum Cow parsnip Apiaceae White Lomatium utriculatum Spring gold Apiaceae Yellow Oenanthe sarmentosa Water parsley Apiaceae White Osmorhiza chilensis Mountain sweet-cicely Apiaceae Grn/White Sanicula bipinnatifida Purple sanicle Apiaceae Purple Sanicula crassicaulis Pacific sanicle Apiaceae Yellow Sium suave Water-parsnip Apiaceae White Ilex aquifolium* English holly Aquifoliaceae Holly Family White Lysichiton americanum Skunk cabbage Araceae Yellow Hedera helix* English ivy Araliaceae White Achillea millifolium Yarrow Asteraceae White Adenocaulon bicolor Pathfinder Asteraceae White Ambrosia chamissonis Silver bursage Asteraceae Yellow Anaphalis margaritacea Pearly everlasting Asteraceae White Arctium minus* Common burdock Asteraceae Pink Artemesia campestris Northern wormwood Asteraceae Yellow Bellis perennis* English daisy Asteraceae White/yel Chrysanthemum leucanthemum* Ox-eye daisy Asteraceae White/yel Cirsium arvense* Canada thistle Asteraceae Pink Cirsium brevistylum Indian thistle -

Irish Plant Monitoring Scheme 2016 Pilot Grasslands Learn Plant
Irish Plant Monitoring Scheme 2016 Pilot Grasslands Help track changes in Ireland’s Flora Learn plant identification and help track changes in Ireland’s Flora Introduction What is it? The Irish Plant Monitoring Scheme has been developed so that we can better understand our environment and track changes in Ireland’s Flora. The scheme sets out to encourage, support and co-ordinate volunteers interested in botanical recording. It is also a fantastic learning experience, especially for beginners as you will be provided with ID guides, a structured way to record and a learning support system. Why should I take part? By taking part in the Irish Plant Monitoring Scheme you will be contributing to a national recording scheme, which will go on to help track changes in Ireland’s Flora. There is ample opportunity for you to upskill and learn more about plants, particularly if you are a beginner to botany. There is also a great opportunity for volunteers who are already familiar with wildflowers to learn new groups (grasses, sedges, etc.). The scheme enables you to claim a 1km² site to record within, there is potential for you to track changes (species, habitats, threats, etc.) in these sites into the future. This is particularly useful if you already regularly record at the site but aren’t making comprehensive lists of species, habitat type or potential threats. Methodology The entire scheme is managed using an online system at: (https://surveys.biodiversityireland.ie) This site allows you claim your survey site and is where you will submit your data. • Request 1km² site using online system (https://surveys.biodiversityireland.ie) • You can choose between a Priority site, Additional site or your own Elected site. -
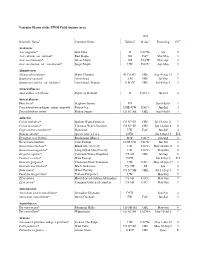
Cedarburg Bog Plants List
Vascular Plants of the UWM Field Station Area Wet Scientific Name1 Common Name Habitat2 Status3 Flowering CC4 Aceraceae Acer negundo* Box Elder D FACW- Apr 0 Acer rubrum var. rubrum* Red Maple SH FAC Mar-May 3 Acer saccharinum* Silver Maple SH FACW Mar-Apr 2 Acer saccharum var. saccharum* Sugar Maple UW FACU Apr-May 5 Alismataceae Alisma subcordatum Water-Plantain M CS SH OBL Aug 4-Aug 21 3 Sagittaria cuneata* Arrowhead A M OBL Jul-Sep 7 Sagittaria latifolia var. latifolia* Arrowhead, Wapato A M SC OBL Jul 4-Sep 5 3 Amaranthaceae Amaranthus retroflexus Pigweed, Redroot D FACU+ Jul-Oct 0 Anacardiaceae Rhus hirta* Staghorn Sumac FD Jun 8-Jul 8 2 Toxicodendron radicans subsp. negundo Poison-Ivy D FD UW FAC+ Jun-Jul 4 Toxicodendron vernix Poison Sumac CS SC SH OBL Jun 8-Jul 8 7 Apiaceae Cicuta bulbifera* Bulblet Water-Hemlock CS SC SH OBL Jul 19-Sep 11 7 Cicuta maculata* Common Water-Hemlock CS SC SH OBL Jun 16-Sep 8 6 Cryptotaenia canadensis* Honewort UW FAC Jun-Jul 4 Daucus carota* Queen Anne's-Lace D FD Jul 4-Sep 24 E:I Eryngium yuccifolium Rattlesnake-Master FD P FAC+ Jul-Aug 8 Heracleum lanatum Cow-Parsnip D SH UW FACW Jun-Jul 3 Osmorhiza claytonii* Bland Sweet Cicely UW FACU- May 24-Jun 11 5 Osmorhiza longistylis* Long-Styled Sweet Cicely UW FACU- May-Jun 4 Oxypolis rigidior* Common Water-Dropwort CS SH OBL Jul-Sep 6 Pastinaca sativa* Wild Parsnip D FD Jun 8-Sep 2 E:I Sanicula gregaria* Clustered Black Snakeroot UW FAC+ May 26-Jun 29 3 Sanicula marilandica* Black Snakeroot CS UW NI Jun 5 Sium suave* Water-Parsnip CS SC SH OBL Jul 14-Sep 5 5 Taenidia integerrima* Yellow-Pimpernel UW May-Jun 7 Zizia aptera Heart-Leaved Golden Alexanders CS SH FACU May-Jun 9 Zizia aurea* Common Golden Alexanders CS SH FAC+ May-Jun 7 Apocynaceae Apocynum androsaemifolium* Spreading Dogbane D FD Jun-Sep 2 Apocynum cannabinum* Hemp-Dogbane FW FAC Jun-Jul 3 Aquifoliaceae Ilex mucronata* Mountain Holly CS OB SH OBL May-Jun 8 Ilex verticillata* Winterberry CS SC SH FACW+ May 10-Jul 5 7 Araceae Arisaema triphyllum subsp.