Effects of Hydrogen Peroxide on Common Aviation Textiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
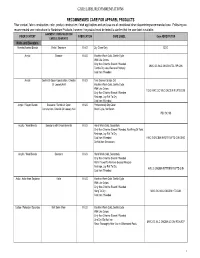
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

The Piedmont Service: Hydrogen Fuel Cell Locomotive Feasibility
The Piedmont Service: Hydrogen Fuel Cell Locomotive Feasibility Andreas Hoffrichter, PhD Nick Little Shanelle Foster, PhD Raphael Isaac, PhD Orwell Madovi Darren Tascillo Center for Railway Research and Education Michigan State University Henry Center for Executive Development 3535 Forest Road, Lansing, MI 48910 NCDOT Project 2019-43 FHWA/NC/2019-43 October 2020 -i- FEASIBILITY REPORT The Piedmont Service: Hydrogen Fuel Cell Locomotive Feasibility October 2020 Prepared by Center for Railway Research and Education Eli Broad College of Business Michigan State University 3535 Forest Road Lansing, MI 48910 USA Prepared for North Carolina Department of Transportation – Rail Division 860 Capital Boulevard Raleigh, NC 27603 -ii- Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. FHWA/NC/2019-43 4. Title and Subtitle 5. Report Date The Piedmont Service: Hydrogen Fuel Cell Locomotive Feasibility October 2020 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Andreas Hoffrichter, PhD, https://orcid.org/0000-0002-2384-4463 Nick Little Shanelle N. Foster, PhD, https://orcid.org/0000-0001-9630-5500 Raphael Isaac, PhD Orwell Madovi Darren M. Tascillo 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Center for Railway Research and Education 11. Contract or Grant No. Michigan State University Henry Center for Executive Development 3535 Forest Road Lansing, MI 48910 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered Final Report Research and Development Unit 104 Fayetteville Street December 2018 – October 2020 Raleigh, North Carolina 27601 14. Sponsoring Agency Code RP2019-43 Supplementary Notes: 16. -

Hydrogen Storage for Mobility: a Review
materials Review Hydrogen Storage for Mobility: A Review Etienne Rivard * , Michel Trudeau and Karim Zaghib * Centre of Excellence in Transportation Electrification and Energy Storage, Hydro-Quebec, 1806, boul. Lionel-Boulet, Varennes J3X 1S1, Canada; [email protected] * Correspondence: [email protected] (E.R.); [email protected] (K.Z.) Received: 18 April 2019; Accepted: 11 June 2019; Published: 19 June 2019 Abstract: Numerous reviews on hydrogen storage have previously been published. However, most of these reviews deal either exclusively with storage materials or the global hydrogen economy. This paper presents a review of hydrogen storage systems that are relevant for mobility applications. The ideal storage medium should allow high volumetric and gravimetric energy densities, quick uptake and release of fuel, operation at room temperatures and atmospheric pressure, safe use, and balanced cost-effectiveness. All current hydrogen storage technologies have significant drawbacks, including complex thermal management systems, boil-off, poor efficiency, expensive catalysts, stability issues, slow response rates, high operating pressures, low energy densities, and risks of violent and uncontrolled spontaneous reactions. While not perfect, the current leading industry standard of compressed hydrogen offers a functional solution and demonstrates a storage option for mobility compared to other technologies. Keywords: hydrogen mobility; hydrogen storage; storage systems assessment; Kubas-type hydrogen storage; hydrogen economy 1. Introduction According to the Intergovernmental Panel on Climate Change (IPCC), it is almost certain that the unusually fast global warming is a direct result of human activity [1]. The resulting climate change is linked to significant environmental impacts that are connected to the disappearance of animal species [2,3], decreased agricultural yield [4–6], increasingly frequent extreme weather events [7,8], human migration [9–11], and conflicts [12–14]. -

H2@Railsm Workshop
SANDIA REPORT SAND2019-10191 R Printed August 2019 H2@RailSM Workshop Workshop and report sponsored by the US Department of Energy Office of Energy Efficiency and Renewable Energy Fuel Cell Technologies Office, and the US Department of Transportation Federal Railroad Administration. Prepared by Mattie Hensley, Jonathan Zimmerman Prepared by Sandia National Laboratories Albuquerque, New MexiCo 87185 and Livermore, California 94550 Issued by Sandia National Laboratories, operated for the United States Department of Energy by National Technology & Engineering Solutions of Sandia, LLC. NOTICE: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. References herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government, any agency thereof, or any of their contractors or subcontractors. The views and opinions expressed herein do not necessarily state or reflect those of the United States Government, any agency thereof, or any of their contractors. Printed in the United States of America. This report has been reproduced directly from the best available copy. Available to DOE and DOE contractors from U.S. Department of Energy Office of Scientific and Technical Information P.O. -

Comparison of Hydrogen Powertrains with the Battery Powered Electric Vehicle and Investigation of Small-Scale Local Hydrogen Production Using Renewable Energy
Review Comparison of Hydrogen Powertrains with the Battery Powered Electric Vehicle and Investigation of Small-Scale Local Hydrogen Production Using Renewable Energy Michael Handwerker 1,2,*, Jörg Wellnitz 1,2 and Hormoz Marzbani 2 1 Faculty of Mechanical Engineering, University of Applied Sciences Ingolstadt, Esplanade 10, 85049 Ingolstadt, Germany; [email protected] 2 Royal Melbourne Institute of Technology, School of Engineering, Plenty Road, Bundoora, VIC 3083, Australia; [email protected] * Correspondence: [email protected] Abstract: Climate change is one of the major problems that people face in this century, with fossil fuel combustion engines being huge contributors. Currently, the battery powered electric vehicle is considered the predecessor, while hydrogen vehicles only have an insignificant market share. To evaluate if this is justified, different hydrogen power train technologies are analyzed and compared to the battery powered electric vehicle. Even though most research focuses on the hydrogen fuel cells, it is shown that, despite the lower efficiency, the often-neglected hydrogen combustion engine could be the right solution for transitioning away from fossil fuels. This is mainly due to the lower costs and possibility of the use of existing manufacturing infrastructure. To achieve a similar level of refueling comfort as with the battery powered electric vehicle, the economic and technological aspects of the local small-scale hydrogen production are being investigated. Due to the low efficiency Citation: Handwerker, M.; Wellnitz, and high prices for the required components, this domestically produced hydrogen cannot compete J.; Marzbani, H. Comparison of with hydrogen produced from fossil fuels on a larger scale. -

Fuel Cell Power Spring 2021
No 67 Spring 2021 www.fuelcellpower.wordpress.com FUEL CELL POWER The transition from combustion to clean electrochemical energy conversion HEADLINE NEWS CONTENTS Hydrogen fuel cell buses in UK cities p.2 The world’s first hydrogen fuel cell Zeroavia’s passenger plane flight p.5 double decker buses have been Intelligent Energy’s fuel cell for UAV p.6 delivered to the City of Aberdeen. Bloom Energy hydrogen strategy p.7 Wrightbus is following this up with Hydrogen from magnesium hydride paste p.11 orders from several UK cities. ITM expanding local production of zero emission hydrogen p.12 The Scottish Government is support- Alstom hydrogen fuel cell trains p.13 ing the move to zero emission Zero carbon energy for emerging transport prior to the meeting of World markets p.16 COP26 in Glasgow later this year. Nel hydrogen infrastructure p.18 Australia’s national hydrogen strategy p.20 The United Nations states that the Ballard international programmes p.22 world is nowhere close to the level FuelCell Energy Government Award p.26 of action needed to stop dangerous Ulemco’s ZERRO ambulance p.27 climate change. 2021 is a make or Adelan fuel cells in UK programme p.28 break year to deal with the global Wilhelmsen zero emission HySHIP p.29 climate emergency. Hydrogen fuel cell yacht p.30 NEWS p.10 EVENTS p.30 HYDROGEN FUEL CELL BUSES IN UK CITIES WORLD’S FIRST in tackling air pollution in the city.” Councillor Douglas Lumsden added: “It is HYDROGEN FUEL CELL fantastic to see the world’s first hydrogen- DOUBLE DECKER BUS IN powered double decker bus arrive in ABERDEEN Aberdeen. -

Nylon Wool Fiber Columns
U.S. Corporate Headquarters Polysciences Europe GmbH Polysciences Asia-Pacific, Inc. 400 Valley Rd. Badener Str. 13 2F-1, 207 DunHua N. Rd. Warrington, PA 18976 69493 Hirschberg an der Taipei, Taiwan 10595 1(800) 523-2575 / (215) 343-6484 Bergstrasse, Germany (886) 2 8712 0600 1(800)343-3291 fax +(49) 6201 845 20 0 (886) 2 8712 2677 fax [email protected] +(49) 6201 845 20 20 fax [email protected] [email protected] TECHNICAL DATA SHEET 425A Page 1 of 2 Nylon Wool Fiber Columns BACKGROUND NYLON WOOL FIBER VS. SHEEP RBC ROSETTING METHODS Researchers have been using nylon wool fiber procedures to separate T-cell and Wong and Mittal (1981)9 did extensive research comparing the methods of Nylon B-cell lymphocytes for more than 20 years. In the early 1970’s M. H. Julius et al Wool Fiber separation and the commonly- used and well-studied sheep RBC (1973),1 Eisen et al (1972),2 and Greaves & Brain (1974)3 described specific (SRBC) rosetting.10,11 Wong and Mittal were interested in isolating B-cells for conditions for the use of Nylon Wool Fiber in columns or plastic straws. These serologic typing of HLA-DR antigen. protocols resulted in yields of 50-90% T-cell recovery and 10-100 fold B-cell depletion. Wong and Mittal concluded that “Due to its simplicity and reliability, nylon wool adherence may be preferred over the SRBC rosette method for the routine pheno- These early researchers found it necessary to scrub or wash their Nylon Wool Fiber typing of B-cells.” Their findings are illustrated in Table 1. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Conversion of a Gasoline Internal Combustion Engine to a Hydrogen Engine
Paper ID #3541 Conversion of a Gasoline Internal Combustion Engine to a Hydrogen Engine Dr. Govind Puttaiah P.E., West Virginia University Govind Puttaiah is the Chair and a professor in the Mechanical Engineering Department at West Virginia University Institute of Technology. He has been involved in teaching mechanical engineering subjects during the past forty years. His research interests are in industrial hydraulics and alternate fuels. He is an invited member of the West Virginia Hydrogen Working Group, which is tasked to promote hydrogen as an alternate fuel. Timothy A. Drennen Timothy A. Drennen has a B.S. in mechanical engineering from WVU Institute of Technology and started with EI DuPont de Nemours and Co. in 2010. Mr. Samuel C. Brunetti Samuel C. Brunetti has a B.S. in mechanical engineering from WVU Institute of Technology and started with EI DuPont de Nemours and Co. in 2011. Christopher M. Traylor c American Society for Engineering Education, 2012 Conversion of a Gasoline Internal Combustion Engine into a Hydrogen Engine Timothy Drennen*, Samual Brunetti*, Christopher Traylor* and Govind Puttaiah **, West Virginia University Institute of Technology, Montgomery, West Virginia. ABSTRACT An inexpensive hydrogen injection system was designed, constructed and tested in the Mechanical Engineering (ME) laboratory. It was used to supply hydrogen to a gasoline engine to run the engine in varying proportions of hydrogen and gasoline. A factory-built injection and control system, based on the injection technology from the racing industry, was used to inject gaseous hydrogen into a gasoline engine to boost the efficiency and reduce the amount of pollutants in the exhaust. -

FACT SHEET 7: Liquid Hydrogen As a Potential Low- Carbon Fuel for Aviation
FACT SHEET 7: Liquid hydrogen as a potential low- carbon fuel for aviation This fact sheet aims to explain how current aviation fuels operate before providing descriptions of how alternative fuel options, like sustainable aviation fuels (SAF) and liquid hydrogen, could help meet the rigorous climate targets set by the aviation industry. Secondly, this document explores the limitations and opportunities of liquid hydrogen when it comes to the manufacturing, safety, current uses and outlooks. This document concludes with a discussion on policy, mandates and incentives on the topic of hydrogen as a potential fuel for aviation. Introduction – Why hydrogen? Aircraft fly thanks to a combination of air and a combustion process that occurs in the aircraft engines. The primary source of energy is the fuel. Each kilogram of fuel, which would occupy less than 1 litre of volume, contains a significant amount of energy, 42.8 MJ [1]. If we could convert the energy of a 1L bottle of fuel into electric energy to power a cell phone, the battery would last for over 2 months. This energy is extracted in the combustion chamber of the engine in the form of heat; Compressed air enters the combustion chamber and gets heated up to temperatures nearing 1,500°C. This hot high- pressure air is what ultimately moves the aircraft forward. Kerosene is composed of carbon and hydrogen (hence it’s a hydrocarbon fuel). When the fuel is completely burned, these carbon and hydrogen molecules recombine with oxygen to create water vapor (H2O) and carbon dioxide (CO2) (Fig.1). August 2019 Emissions: From Combustion: H2O + CO2 Other: NOx, nvPM SOx, soot Fuel in (C + H) Figure 1 Schematic of a turbofan engine adapted from: [2] Carbon dioxide will always be created as a by-product of burning a carbon-based fuel.