Nylon Wool Fiber Columns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Care Label Recommendations
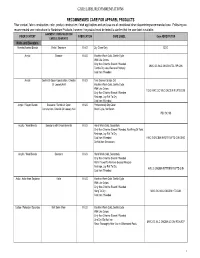
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

Wool Is 100% Biodegradable
WOOL FACTS WOOL IS 100% BIODEGRADABLE Wool is a natural and renewable resource. As long as there is grass to eat, sheep will continue to produce wool. When wool is disposed of, it will naturally decompose in soil in a matter of months or years, slowly releasing valuable nutrients back into the earth. Synthetic fibres, on the other hand, can be extremely slow to degrade and significantly contribute to the world’s overflowing landfills. BIODEGRADATION N, S & other OF WOOL nutrients All materials of animal and vegetable origin have some degree HOW DOES of biodegradability, meaning that they are capable of being WOOL decomposed by the action of living organisms, such as fungi BIODEGRADE? and bacteria. Wool is composed of the natural protein keratin, which is similar to the protein that makes up human hair. When keratin is broken down naturally by microorganisms, the products do not pose any environmental hazard. On disposal, if wool is kept warm and moist or buried in soil, WOOL READILY fungal and bacterial growths develop which produce enzymes that BIODEGRADES digest wool. IN MOIST, WARM On the other hand, thanks to the unique chemical structure of keratin and wool’s tough, water-repellent outer membrane, clean and dry CONDITIONS wool fibres do not readily degrade. This allows wool products to be resilient and long-lasting in normal conditions. WOOL IS 100% BIODEGRADABLE WOOL BIODEGRADES QUICKLY Wool biodegrades readily in as little as three to four months but the rate varies with soil, climate and wool characteristics. This releases essential elements such as nitrogen, sulphur and magnesium back to the soil, able to be taken up by growing plants. -

Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1991 Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Tao, Weiying, "Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers." (1991). LSU Historical Dissertations and Theses. 5213. https://digitalcommons.lsu.edu/gradschool_disstheses/5213 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Immersion Dyeing Nylon and Acetate Rayon Using Prosperse Disperse Dyes Please Read Directions Carefully Before Starting
Immersion Dyeing Nylon and Acetate Rayon using PROsperse Disperse Dyes Please read directions carefully before starting. For medium to dark shades, it is recommended that nylon be dyed with acid dyes, because disperse dyes lack acceptable fastness. Acetate rayon can only be dyed with disperse dyes and has acceptable fastness in all depths of shade with the disperse dyes. All Dyeing should be done in a stainless steel or enamelware container only. Never use aluminum pots. Use Pyrex or stainless steel measuring utensils and a large wooden dowel for stirring in the boiling dye bath. Always do test samples before working on a large project. Please Note: These dyes have the potential to stain any sink that is not made of stainless steel or fireclay ceramic. For additional information, visit our web site at www.prochemicalanddye.com. Wear rubber gloves, apron, or old clothes. Utensils used for dyeing should never be used for food preparation. Supplies PROsperse Disperse Dye Citric Acid Crystals or White Distilled Vinegar Synthrapol PRO Dye Activator or Soda Ash Procedure 1. Scour the fabric by machine washing in HOT 140F (60C) water, or by hand in a pot on the stove with 2 tsp (2 gm) PRO Dye Activator or Soda Ash and 2 tsp (2.5 ml) Synthrapol per pound of fabric (454 gm, or 3 to 4 yards of muslin weight fabric). Rinse thoroughly. This step does not add the dye fixative to the fabric; it prepares your fabric for dyeing by removing any dirt, oil or sizing. 2. Dissolve the dye. Thoroughly dissolve the desired amount of dye powder, from the chart below, in 1 cup (250 ml) of boiling water. -

Pashmina Wool–A Valuable Commodity
International Journal of Avian & Wildlife Biology Mini Review Open Access Pashmina wool–a valuable commodity Abstract Volume 3 Issue 6 - 2018 The conversion of goat hair into Pashmina was investigated. Pashmina is obtained Herbert W Ockerman from the Changthangi goats found in the Himalayan regions. The nomadic herders and The Ohio State University, USA animals that live in these regions have to adapt to harsh environments. The Pashmina goats play an important role in the livelihoods of the nomadic herders. Correspondence: Herbert W Ockerman, The Ohio State University, Ohio, USA, Email Keywords: changthangi, pashmina, goat, cashmere, himalayas, ladakh Received: November 01, 2018 | Published: November 16, 2018 Introduction The study investigated the ethnozoological aspects of agriculture in hostile environments and the production of the finest wool in the world. Changthangi or Pashmina goats can tolerate high altitude and the harsh environment of the Himalayan desert by growing an undercoat of fine hair which serves as insulation to keep them warm. This is the origin of pashmina wool. The research showed that the animals found in these regions such as yak, sheep and goats play a critical role in allowing humans to exist in a harsh environment. The elevation of these regions is upwards of 4,350 m (14,270 ft.) which causes a lack of oxygen, cold temperatures ranging from –20°C (–4°F) to –40°C (–40°F), strong winds, meager rainfall and lack of vegetation. This report will focus on the domestic Changthangi (or Pashmina) breed which produces wool that is known for its firmness, warmth, durability, lightness, softness and ability to Figure 2 Pashmina goat, sheep and yak herding. -

Colaris Digital Textile Printing
ZIMMER AUSTRIA | DIGITAL PRINTING SYSTEMS COLARIS DIGITAL TEXTILE PRINTING HOME TEXTILES APPAREL DECORATION AUTOMOTIVE FLAGS & BANNERS www.zimmer-austria.com 2020.01.15 page 1 CONTENT 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES 3 2. INK CLASSES 2.1. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 4 2.2. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 5 3. PRINT TECHNOLOGY 3.1. PROCESSING DIAGRAM 6 3.2. PROCESS EQUIPMENT 7 4. REACTIVE PRINTING 4.1. GENERAL INFORMATION 8 4.2. EXAMPLE: TERRY TOWEL PRINT PRODUCTION 9 5. ACID PRINTING 5.1. GENERAL INFORMATION 10 5.2. EXAMPLE: UPHOLSTERY PRINT LINE 11 6. DISPERSE / SUBLIMATION PRINTING 6.1. GENERAL INFORMATION 12 6.2. EXAMPLE: PES BLANKET PRINT LINE 13 7. VAT INDANTHRENE® PRINTING 7.1. GENERAL INFORMATION 14 7.2. APPLICATION DIVERSITY 15 8. PIGMENT PRINTING 8.1. GENERAL INFORMATION 16 8.2. APPLICATION DIVERSITY 17 9. CATIONIC PRINTING 9.1. GENERAL INFORMATION 18 10. COLARIS - CHARACTERISTICS AND FEATURES 10.1. COLARIS MODELS 19 11. COLARIS FEATURES AND COMPONENTS 11.1. INTEGRATED MACHINE COMPONENTS 20 11.2. INTEGRATED MACHINE COMPONENTS 21 12. PROCESS EQUIPMENT 12.1. INLINE COMPONENTS 22 12.2. OFFLINE COMPONENTS 23 13. PRINT HEAD 13.1. TECHNOLOGY 24 13.2. RECONDITION CENTER 25 14. ZIMMER TECHNOLOGY & APPLICATION CENTER 14.1. GENERAL INFORMATION 26 14.2. EQUIPMENT & FACILITIES 27 www.zimmer-austria.com 2020.01.15 page 2 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES Vertical Duplex blanket printer from 1951 First commercial rotary screen printer 1958 The broad digital competence of ZIMMER AUSTRIA is based on an innovation introduced more than 4 decades ago. -

FABRICS/ DYING Dictionary
FABRICS/ DYING dictionary ACRYLIC BABYCORD Acrylic fabric is a manufactured fiber with a soft wool-like feel and Babycord is a ribcord fabric with a very small and thin rib line. The an uneven finish. It is used widely in knits as the fabric has the same fabric is often lighter and softer than normal or corduroy fabric. It is cozy look as wool. Acrylic fabric is favored for a variety of reasons very soft and comfortable, and is often made in a stretch quality. it is warm, quite soft, holds color well, is both stain and wrinkle resistant and it doesn’t itch. These qualities make acrylic a great BLEND substitute for wool. A blend fabric or yarn is made up of more than one fibre. In the yarn, two or more different types of fibres are used to form the yarn. ALPACA Blends are used to create a more comfortable fabric with a softer Alpaca wool comes from a South American animal that roams the feel. A good example is a cotton/wool blend; the mixture of cotton mountain slopes of Ecuador, Peru, Bolivia and Chile. The fleece and wool will prevent the fabric from being excessively warm and from an alpaca is similar to wool or mohair, but is softer, silkier, and will make the fabric softer to the skin. warmer. Because alpaca wool takes much longer to grow it is often more expensive and exclusive. However, garments made from this BOUCLE fabric are stronger and more comfortable. The term boucle is derived from the French word boucle, which literally means “to curl”. -

Sea Silk and Shellfish Purple Dye in Antiquity, Ed. HL Enegren and F
Fulcher, K 2017 Review of Treasures from the Sea: Sea Silk and Shellfish Purple Dye in Antiquity, ed. H. L. Enegren and F. Meo. Papers from the Institute of Archaeology, 27(1): Art. 15, pp. 1–4, DOI: https://doi.org/10.5334/pia-535 REVIEW Review of Treasures from the Sea: Sea Silk and Shellfish Purple Dye in Antiquity, ed. H. L. Enegren and F. Meo Kate Fulcher Treasures from the Sea: Sea silk and shellfish purple dye in antiquity, ed. H. L. Enegren and F. Meo, Oxford; Havertown: Oxbow Books, 224 pages (Hardbound), £38, US$55, 2017, ISBN: 978-1785704352. This volume presents the proceedings of a conference in Lecce in 2013, which brought together several different approaches including archaeology, experimentation, scientific analysis, and terminology. This interdisciplinary approach is reflected in the publication, which both maintains the reader’s interest and works well for ancient materials. This volume presents the proceedings of a 80% during processing. The natural col- conference held in Lecce, Italy, in 2013, on our is a greenish brown, transformed into a the subject of two sea “treasures”: the beard golden brown by cleaning and treating with fibres of the fan mussel, used to weave small lemon juice. It continued to be produced scale textiles known as sea silk, and purple through to modern times, until 1992 when dye extracted from the hypobranchial glands it was placed under the protection of the of certain molluscs. Sixteen papers are pre- EU Habitat Directive. One interesting short sented, the first half focussing on sea silk, and article (Pes & Pes) introduces the reader to the second half on purple dye. -

Dyeing Methods for Wool Blends Contemporary Wool Dyeing and Finishing
Dyeing methods for wool blends Contemporary wool dyeing and finishing Mr Arthur Fisher CSIRO Summary 1. Introduction 2. Dyeing wool/polyester blends 3. Dyeing wool/polyamide blends 4. Dyeing wool acrylic blends 5. Dyeing wool silk blends 6. Dyeing wool cotton blends 1. Introduction Dyeing fibre blends There are three significant reasons for using blends of fibres: § Economy - The partial replacement of expensive fibres, e.g. wool with cheaper fibres, can make the market for a fabric wider, and increase production volumes. § Physical properties - The ability to gain some of the advantages of each fibre can be of significant advantage e.g. polyester can contribute strength and wool moisture absorbency to a polyester/wool blend. § Aesthetics - The attractiveness of the appearance and the handle of the fabric can be improved by the use of blends to give multicoloured fabrics, and combinations of yarns with different characteristics of lustre, crimp or denier. 2. Dyeing wool/polyester blends Dyeing wool/polyester blends § PES/wool blended fabrics are mainly used for apparel, i.e. suits. Blending wool with PES makes the fabric cheaper and increases durability and wrinkle-resistance. Main outlets are worsted fabrics. § The most common blend ratio for PES/WO is 55:45 but a large variety of other blend ratios can also be found in the market. § PES/WO blends are dyed in piece form (solid shades) or as yarn on packages (for pattern wovens). Dyeing wool/polyester blends (cont.) § There are a number of methods by which wool/polyester blends may be dyed, and many dye manufacturers offer products which may be used. -

Fabric Fiber Content
Fabric Types, Count & Fiber Content Zweigart Linen Count Content Belfast 32 100% linen Afghans - 100% Polyacrylic Cashel 28 100% linen Abby 18ct Alba 14ct Almanac 14ct Cork 19 100% linen Anne Cloth 18ct Baby Snuggle 18ct Country Home 18ct Dublin 25 100% linen Diamond 18ct Gloria 14ct Hearthside 14ct Edinborough 36 100% linen Honeycomb 18ct Novara 14ct Patrice 14ct Fine Linen 45 55% linen + 45% cotton Afghans - 100% Cotton Glasgow 28 100% linen Anne Cloth 18ct Augusta 14 ct Novara 14ct Kingston 50 100% linen Teresa 14ct Newcastle 40 100% linen Afghans- Misc Normandie 55% cotton + 45% linen Pastel LinenD 28 52% cotton + 48% linen Gloria 14ct 70% rayon + 30% linen Pearl Linen 20, 25, 28 60% polyester + 40% linen Merino 28ct 100% Wool Mosaik 18ct 52% cotton + 42% rayon Patterned Count Content Tannenbaum 18ct 52% cotton + 42% rayon Cottage Huck 14 100% cotton Aida Weave Count Content Belinda 20 52% cotton + 48% rayon Diana 20 52% cotton + 48% rayon Aida 8, 11, 14, 16, 18 100% cotton Newport 28 100% linen Country AidaD 7 100% polyacrylic Sambuca 28 60% polyester + 40% linen Damask Aida 11,14,18 52% cotton + 48% rayon Saronno 28 52% cotton + 48% rayon GoldauD 7 55% rayon + Shenandoah 28 55% linen + 45% rayon 40% cotton + 5% metallic Hardanger 22 100% cotton Canvas Count Content Hearthstone 14 60% cotton + 40% linen Congress 24 100% cotton Herta 6 100% cotton Congressa 24 100% cotton Huck 14 100% cotton Cordova 22 100% cotton Klostern 7 60% rayon + 40% cotton Double Mesh 5, 6.5, 7.5, 10, 12, Linen Hardanger 22 100% linen 14, 16, 18, 20 100% cotton