(Arachnida: Araneae) of the Galápagos Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Untangling the Web… Spiders in Arizona Fields! Ayman Mostafa, Lydia M
Untangling the Web… Spiders in Arizona Fields! Ayman Mostafa, Lydia M. Brown, Tim Vandervoet, Peter C. Ellsworth (University of Arizona), Vonny Barlow (University of California) & Steven E. Naranjo (USDA-ARS, ALARC) Spiders are beneficial inhabitants of agricultural fields because of Lygus nymph prey their important contributions to biological control of pest insects, consuming tons of small arthropods every year. Spiders eat anything they can catch, even prey larger than themselves. When they are abundant, they contribute to the control of many insect pests in A Arizona crop fields including whiteflies, Lygus bugs, fleahoppers, Leafhopper and lepidopteran larvae. Field studies in Arizona demonstrate that the prey B crab spider, Misumenops celer (Family Thomisidae, Fig. 1A, B) and Dictyna spider, Dictyna reticulata (Family Dictynidae, Fig. 1C, D) are common in Arizona cotton fields and can be influential predators. Unlike other spiders that spin webs to capture their food, crab spiders rely on stealth and surprise. They actively search plant surfaces, litter, and debris for prey. They hide in flowers or foliage and ambush their prey. Their common name derives from the fact that they look like and walk like crabs. Dictyna are small, brownish, web-making E spiders that trap whitefly adults and other insects in their webs (Fig. 1C). Examining their webs enables easy identification of what D species of whitefly are in the field (sweetpotato or banded-winged). C Jumping spiders (Family Salticidae, Fig. 1E) are generally less abundant in cotton fields but, like crab spiders, ambush their prey. They have stout bodies and long front legs adapted for jumping, as well as four pairs of eyes with one very large set in the middle of their face. -

Abundance and Community Composition of Arboreal Spiders: the Relative Importance of Habitat Structure
AN ABSTRACT OF THE THESIS OF Juraj Halaj for the degree of Doctor of Philosophy in Entomology presented on May 6, 1996. Title: Abundance and Community Composition of Arboreal Spiders: The Relative Importance of Habitat Structure. Prey Availability and Competition. Abstract approved: Redacted for Privacy _ John D. Lattin, Darrell W. Ross This work examined the importance of structural complexity of habitat, availability of prey, and competition with ants as factors influencing the abundance and community composition of arboreal spiders in western Oregon. In 1993, I compared the spider communities of several host-tree species which have different branch structure. I also assessed the importance of several habitat variables as predictors of spider abundance and diversity on and among individual tree species. The greatest abundance and species richness of spiders per 1-m-long branch tips were found on structurally more complex tree species, including Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco and noble fir, Abies procera Rehder. Spider densities, species richness and diversity positively correlated with the amount of foliage, branch twigs and prey densities on individual tree species. The amount of branch twigs alone explained almost 70% of the variation in the total spider abundance across five tree species. In 1994, I experimentally tested the importance of needle density and branching complexity of Douglas-fir branches on the abundance and community structure of spiders and their potential prey organisms. This was accomplished by either removing needles, by thinning branches or by tying branches. Tying branches resulted in a significant increase in the abundance of spiders and their prey. Densities of spiders and their prey were reduced by removal of needles and thinning. -
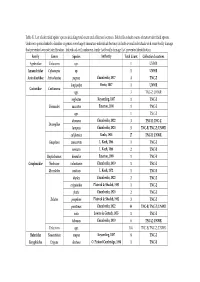
Table S1. List of Identified Spider Species Including Total Count and Collection Locations. Bold Cells Include Counts of Mature Identified Species
Table S1. List of identified spider species including total count and collection locations. Bold cells include counts of mature identified species. Unknown species linked to families or genera were largely immature individuals but may include several individuals with some bodily damage that prevented accurate identification. Individuals with unknown family had bodily damage that prevented identification. Family Genus Species Authority Total Count Collection Locations Agelenidae Unknown spp. 1 UNWR Amaurobiidae Cybaeopsis sp. 1 UNWR Antrodiaetidae Antrodiaetus pugnax Chamberlin, 1917 4 TNC-Z longipalpa Hentz, 1847 1 UNWR Corinnidae Castianeira spp. 3 TNC-Z; UNWR neglectus Keyserling, 1887 1 TNC-Z Drassodes saccatus Emerton, 1890 1 TNC-Z spp. 1 TNC-Z dromeus Chamberlin, 1922 3 TNC-B; TNC-Z Drassyllus lamprus Chamberlin, 1920 3 TNC-B; TNC-Z; UNWR californica Banks, 1904 17 TNC-B; UNWR Gnaphosa muscorum L. Koch, 1866 1 TNC-Z sericata L. Koch, 1866 2 TNC-B Haplodrassus hiemalis Emerton, 1909 1 TNC-B Gnaphosidae Nodocion voluntaries Chamberlin, 1919 1 TNC-Z Urozelotes rusticus L. Koch, 1872 1 TNC-B duplex Chamberlin, 1922 2 TNC-Z exiguioides Platnick & Shadab, 1983 1 TNC-Z fratis Chamberlin, 1920 2 TNC-Z Zelotes josephine Platnick & Shadab, 1983 3 TNC-Z puritanus Chamberlin, 1922 44 TNC-B; TNC-Z; UNWR sula Lowrie & Gertsch, 1955 1 TNC-Z tubuous Chamberlin, 1919 6 TNC-Z; UNWR Unknown spp. 184 TNC-B; TNC-Z; UNWR Hahniidae Neoantistea magna Keyserling, 1887 8 TNC-Z Linyphiidae Erigone dentosa O. Pickard-Cambridge, 1894 1 TNC-B spp. 1 TNC-Z Unknown spp. 13 TNC-Z; UNWR mccooki Montgomery, 1904 95 TNC-B; TNC-Z; UNWR Schizocosa minnesotensis Gertsch, 1934 25 TNC-B Lycosidae spp. -

Mimicry and Camouflaging Spiders
Antelope Island Spider Festival Mimicry and Camouflaging Spiders In the natural world mimicry is a phenomenon These spiders will mimic ants in several encountered frequently in the natural world, different ways. Anything from moving in the and the world of spiders is no exception. same fashion as ants to even having altered body shapes. One species, Castianeira Mimicry can be used by one organism to avoid longipalpa (kass-tee-uh-NEE-ruh lon-jih-PAHL- predation by puh), even goes so far as to wave around its looking or front two legs like antennae. acting similar to a more For the most part spiders in the Corinnidae dangerous family don’t spin a web with the exception of a organism. Such small shelter, called a sac, to rest in underneath as with the a log or rock or in the leaf litter when they scarlet king aren’t hunting for food or looking for mates. snake, which is Corinnidae spiders tend to prey on smaller harmless, and insects. Some common prey items include ants, the eastern ant larvae, leaf or tree hoppers, fruit flies, and coral snake, micro moths. which is highly venomous. Another phenomenon found frequently in the natural world is camouflage, and once again Mimicry can also be used aggressively, as in a spiders utilize this as well. predator resembling its prey, or a parasite its host. There are several examples of spiders Camouflage can be used by an organism to performing this kind of mimicry. blend in with its background to avoid predation. It can also be used by an ambush There are at least two families of spiders with predator to blend in with the background so members that practice mimicry. -

Note on Suspected Brown Recluse Spiders (Araneae: Sicariidae) in South Carolina
Faculty Research Note Note on Suspected Brown Recluse Spiders (Araneae: Sicariidae) in South Carolina Robert J. Wolff* South University, 9 Science Court, Columbia, SC 29203 The general public believes that brown recluse spiders (Loxosceles Filistatidae (Kukulcania hibernalis) 22 specimens reclusa) are widespread where they live and that these spiders are Lycosidae 21 (3 in one package, 5 in another) frequent causes of bites resulting in dermonecrosis. Research over the Pholcidae 17 past twenty years shows these reports to be unfounded. Vetter (2005) Miturgidae 8 examined 1,773 specimens sent in from across the U.S. as brown recluse Theridiidae 8 spiders and no specimens were found from areas outside the species Agelenidae 7 range, with the exception of a specimen from California. Araneidae 6 Clubionidae 6 The reported range of the brown recluse spider includes all or major Thomisidae 6 portions of Arkansas, Oklahoma, Texas, Louisiana, Alabama, Tennessee, Gnaphosidae 4 Kentucky, Illinois, Missouri and Kansas. Minor portions of the brown Corinnidae 3 recluse range were previously reported in Iowa, Indiana, Ohio, New Philodromidae 3 Mexico, North Carolina, Georgia, and South Carolina. The most recent Amaurobiidae 1 map (Vetter, 2015) does not include South Carolina, and only the far Pisauridae 1 western tip of North Carolina and northwestern corner of Georgia. Scytodidae (Scytodes thoracica) 1 Unidentifiable 4 Schuman and Caldwell (1991) found that South Carolina physicians reported treating 478 cases of brown recluse spider envenomations in 1990 alone. This seems like a very high number, unfortunately all or No brown recluses were identified from the specimens obtained in this almost all of these are probably not brown recluse spider bites. -

SA Spider Checklist
REVIEW ZOOS' PRINT JOURNAL 22(2): 2551-2597 CHECKLIST OF SPIDERS (ARACHNIDA: ARANEAE) OF SOUTH ASIA INCLUDING THE 2006 UPDATE OF INDIAN SPIDER CHECKLIST Manju Siliwal 1 and Sanjay Molur 2,3 1,2 Wildlife Information & Liaison Development (WILD) Society, 3 Zoo Outreach Organisation (ZOO) 29-1, Bharathi Colony, Peelamedu, Coimbatore, Tamil Nadu 641004, India Email: 1 [email protected]; 3 [email protected] ABSTRACT Thesaurus, (Vol. 1) in 1734 (Smith, 2001). Most of the spiders After one year since publication of the Indian Checklist, this is described during the British period from South Asia were by an attempt to provide a comprehensive checklist of spiders of foreigners based on the specimens deposited in different South Asia with eight countries - Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka. The European Museums. Indian checklist is also updated for 2006. The South Asian While the Indian checklist (Siliwal et al., 2005) is more spider list is also compiled following The World Spider Catalog accurate, the South Asian spider checklist is not critically by Platnick and other peer-reviewed publications since the last scrutinized due to lack of complete literature, but it gives an update. In total, 2299 species of spiders in 67 families have overview of species found in various South Asian countries, been reported from South Asia. There are 39 species included in this regions checklist that are not listed in the World Catalog gives the endemism of species and forms a basis for careful of Spiders. Taxonomic verification is recommended for 51 species. and participatory work by arachnologists in the region. -

Araneae : Philodromidae and Thomisidae, by Charles D
The Journal of Arachnology 8 : 95 Muchmore, W . B. 1969a. The pseudoscorpion genus Neochthonius Chamberlin (Arachnida , Chelonethida, Chthoniidae) with description of a cavernicolous species, Amer. Midl . Nat . 81 :387-394 . Muchmore, W. B . 1969b . New species and records of cavernicolous pseudoscorpions of the genu s Microcreagris (Arachnida, Chelonethida, Neobisiidae, Ideobisiinae) . Amer . Mus. Novitate s 2392:1-21 . Muchmore, W. B . 1976 . New species of Apochthonius, mainly from caves in central and eastern United States (Pseudoscirpionida, Chthoniidae) . Proc. Biol . Soc . Washington 89 :67-80 . Muchmore, W . B. and E. M . Benedict . 1976 . Redescription of Apochthonius moestus (Banks), type of the genus Apochthonius Chamberlin (Pseudoscirpionida, Chthoniidae) . J. New York Entomol. Soc. 84 :67-74 . Schuster, R . O . 1966 . New species of Apochthonius from western North America . Pan-Pacifi c Entomol . 42 :178-183 . Manuscript received March 1979, revised May 1979. BOOK REVIEW The Crab Spiders of Canada and Alaska : Araneae : Philodromidae and Thomisidae, by Charles D . Dondale and James H. Redner, 1978 . Part 5 of The Insects and Arachnida o f Canada, Publication 1663, pp . 1-255, 725 figs., 66 maps . Available from Printing and Publishing Supply and Services Canada, Hull, Quebec, Canada K1A OS9, Canada . Canada : $7 .50 ; other countries : $9.00. This handsome, soft-bound volume, dealing with the spider families Philodromidae an d Thomisidae from north of the United States, is a striking contribution to Canadia n arachnology . About half of the taxa of all temperate North America (110 of some 22 3 species) occur in Canada . The authors have drawn much of the data from their many papers on these spiders, but this has been materially supplemented by new appraisals , illustrations, and distribution information . -

Genetic Diversity Analysis of Crab Spider (Araneae: Thomisidae) Based on RAPD-PCR
RESEARCH PAPER Zoology Volume : 4 | Issue : 8 | August 2014 | ISSN - 2249-555X Genetic Diversity Analysis of Crab Spider (Araneae: Thomisidae) based on RAPD-PCR KEYWORDS Crab spiders, RAPD, diversity, Amravati Nandkishor Warghat Navin Sharma Assistant professor, Department of Zoology, Arts, Assistant professor, Department of Zoology, Arts and commerce and Science College , Maregaon, Dist- Science College, Pulgaon, Dist- Wardha, Maharashtra. Yavatmal, Maharashtra. Punam Thakur *Mumtaz Baig Associate professor, Department of Zoology, Govt. Research Scholar, Department of Zoology, Govt. Vidarbha Institute of Science and Humanities, Vidarbha Institute of Science and Humanities, Amravati Amravati-444604. MH *Corresponding Author ABSTRACT The current study deals with the genetic diversity of crab spiders using molecular markers. Total scorable bands were produced using six random primers for the 16 species of crab spiders belonging to family thomisidae. Out of all screened primers, OPP 9 produced highest 113 scorable bands, out of which 111 bands were found with 98.28 percent polymorphism. Primer OPA 3 was produced lowest 43 bands with 100 percent polymor- phism. Remaining primers (OPA 2, OPA 4, OPN 16 and OPN 18) showed 100 per cent polymorphism with 60, 60, 81 and 51 bands respectively. Along with the molecular markers, a morphometric analysis was also focus on the phyloge- netic relationship of crab spiders using UPGMA and NJ approach. The present study is the first report from India to describe the genetic relatedness amongst spider using RAPD-PCR. Introduction at 20055’ and 20.93 North latitude 77045’ and 77.75 East Crab spiders are small to large size, with two claws and longitudes at an elevation of 1125 feet. -

Spiders of the Family Thomisidae in Hawaii1
Pacific Insects 12 (4) : 773-864 25 December 1970 SPIDERS OF THE FAMILY THOMISIDAE IN HAWAII1 By Theodore W. Suman2 Abstract: The spider family Thomisidae in the Hawaiian Islands contains 30 species which constitutes approximately 20 % of the Hawaiian spider fauna. All of the species are endemic to the Hawaiian Islands. The 30 species of Hawaiian Thomisidae are grouped into 2 subfamilies and 5 genera. In the subfamily Misumeninae, 17 of the 21 species are placed in the genus Misumenops which has not been previously recorded for the Hawaiian Islands. The genus Synaema contains 1 species, and the endemic genus Mecaphesa contains 3 species. Five species are synonymized and 9 new species are de scribed in this subfamily. In the subfamily Philodrominae, the endemic genus Proernus contains 5 species and the endemic genus Pagiopalus contains 4 species. One genus and 3 species are synonymized, and 1 new species is described in this subfamily. All species are described and 28 of the 30 species are illustrated. Type specimens are missing for the 2 species not illustrated. A key to the subfamilies, genera and species, and data on the distribution and ecology of each species are presented. Information on the biology and phylogeny of the Thomisidae in the Hawaiian Islands is included. The crab-spider family Thomisidae is a moderately large group and is world-wide in distribution. In the Hawaiian Islands, this family consists of 30 species which is ap proximately 20 % of the spider fauna. Karsch (1880) described Diaea kanakana (= Misumenops kanakanus), the first Ha waiian thomisid, from a group of spiders collected by O. -

A Study on the Spider Fauna of Dargaz and Kalat Counties in Razavi Khorasan Province, Iran (Arachnida: Araneae)
BIHAREAN BIOLOGIST 10 (1): 4-7 ©Biharean Biologist, Oradea, Romania, 2016 Article No.: e151204 http://biozoojournals.ro/bihbiol/index.html A study on the spider fauna of Dargaz and Kalat Counties in Razavi Khorasan Province, Iran (Arachnida: Araneae) Hussein SADEGHI1, Malihe AHMADI2, Alireza ZAMANI3,* and Isa JABALEH2 1. Department of Plant Protection, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran. 2. Higher education Institute of Jihad-e Daneshgahi, Kashmar Branch. 3. Department of Animal Biology, School of Biology and Centre of Excellence in Phylogeny of Living Organisms in Iran, College of Science, University of Tehran, Tehran, Iran *Corresponding author, A. Zamani, E-mail: [email protected] Received: 08. February 2015 / Accepted: 11. May 2015 / Available online: 01. June 2016 / Printed: June 2016 Abstract. In a survey investigating the spider fauna of Dargaz and Kalat counties in Razavi Khorasan Province of Iran, 13 families and 21 species were recognized, of which three are new to Iran: Minosia simeonica Levy, 1995, Nomisia negebensis Levy, 1995 and Thanatus atratus Simon, 1875. Also, genus Minosia Dalmas, 1921 is recorded for the first time in Iran. Data on collection localities and distribution of each species, as well as diagnostic morphological characters and figures for the newly recorded taxa are provided. Key words: new records, Thanatus, Minosia, Nomisia, Iran. Introduction Spiders (Araneae), with over 45000 recognized species in the world (World Spider Catalog 2015). Reviewing the literature, Zamani et al. (2015a) gave a checklist of spiders of Iran with about 540 species. Considering the geographic position of the country as a land bridge joining the Palaearctic, Afro- tropical and Oriental zones, and its diverse climate condi- tions and the known spider fauna of adjacent countries, it seems that this number must be much higher than known at present. -

Arboreal Arthropod Predation on Early Instar Douglas-Fir Tussock Moth Redacted for Privacy
AN ABSTRACT OF THE THESIS OF Becky L. Fichter for the degree of Doctor of Philosophy in Entomologypresented onApril 23, 1984. Title: Arboreal Arthropod Predation on Early Instar Douglas-fir Tussock Moth Redacted for privacy Abstract approved: William P. StOphen Loss of early instar Douglas-fir tussock moth( Orgyia pseudotsugata McDunnough) (DFTM) has been found to constitute 66-92% of intra-generation mortality and to be a key factor in inter-generation population change. This death has been attributed to dispersal and to arthropod predation, two factors previously judged more important to an endemic than an outbreak population. Polyphagous arthropod predators are abundant in the forest canopy but their predaceous habits are difficult to document or quantify. The purpose of the study was to develop and test a serological assay, ELISA or enzyme-linked immunosorbent assay, for use as an indirect test of predation. Development of this assay involved production of an antiserum reactive with DFTM but not reactive with material from any coexisting lepidopteran larvae. Two-dimensional immunoelectrophoresis was used to select a minimally cross-reactive fraction of DFTM hemolymph as the antigen source so that a positive response from a field-collected predator would correlate unambiguously with predation on DFTM. Feeding trials using Podisus maculiventris Say (Hemiptera, Pentatomidae) and representative arboreal spiders established the rate of degredation of DFTM antigens ingested by these predators. An arbitrary threshold for deciding which specimens would be considered positive was established as the 95% confidence interval above the mean of controls. Half of the Podisus retained 0 reactivity for 3 days at a constant 24 C. -

Australian Native Bees Avoid Their Spider Predators
by responding to the same floral signals as these honeybees do (Heiling & Herberstein 2004; Heiling et al. 2004). Fur- thermore, it manipulates visual floral signals. While Euro- pean crab spiders appear camouflaged on flowers (Chittka 2001; The´ry & Casas 2002), T. spectabilis produces a Predator–prey coevolution: strong colour contrast in the UV range of the light spec- trum, attracting honeybees (Heiling et al. 2003). This is Australian native bees in line with empirical data showing that bees are attracted to strongly contrasting marks on flowers (Lunau et al. avoid their spider 1996). predators European honeybees did not coevolve with T. spectabilis but were introduced to Australia ca. 200 years ago. By * contrast, native Australian bees that are also captured by A. M. Heiling and M. E. Herberstein T. spectabilis coevolved with this species. We test our pre- Department of Biological Sciences, Macquarie University, North Ryde, diction that native Australian bees evolved anti-predatory NSW 2109, Australia * Author for correspondence ([email protected]). behaviour to avoid their predators, unlike the naive Euro- pean honeybees. Recd 14.10.03; Accptd 10.11.03; Published online 12.01.04 2. MATERIAL AND METHODS Australian crab spiders Thomisus spectabilis Thomisus spectabilis were collected in suburban areas of Brisbane, manipulate visual flower signals to lure introduced Australia. Females reach a body length of ca. 1 cm. Female coloration Apis mellifera. We gave Australian native bees, Aus- varies and we used only white ones in our experiments. Native bees (Austroplebia australis) that were kept in an outdoor hive at the Uni- troplebia australis, the choice between two white versity grounds were transferred into a growth house and trained to daisies, Chrysanthemum frutescens, one of them visit a feeding station (ca.